Fluorescent Phospholipid Labeling Services
High-PurityFor Drug Delivery ResearchPrecision Lipid Tracing & Membrane Analytics
Advance membrane biology, drug delivery, and nanomedicine research with high-performance fluorescent phospholipids engineered for enterprise-scale biotechnology, pharmaceutical, and diagnostic applications. Our fluorescent phospholipid labeling services combine rigorously validated lipid chemistry, controlled fluorophore integration, and analytical-grade purification to deliver highly defined lipid probes with consistent fluorescence, membrane fidelity, and batch-to-batch reproducibility. From phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylglycerol (PG) to cardiolipin and PEGylated phospholipids, we support custom fluorescent lipid solutions optimized for vesicle formulation, membrane trafficking studies, and in vivo biodistribution analysis.
Whether for liposome development, lipid nanoparticle (LNP) characterization, membrane dynamics research, or targeted delivery systems, our fluorescent phospholipids provide reliable signal stability, minimal perturbation of lipid behavior, and compatibility with advanced imaging and quantitative platforms.
What Are Fluorescent Phospholipids?
Fluorescent phospholipids are lipid molecules covalently or structurally modified with fluorophores to enable visualization and quantitative analysis of membrane structure, lipid trafficking, and lipid-based delivery systems. By integrating fluorophores into phospholipid headgroups or acyl chains, these probes allow precise tracking of lipid distribution without significantly altering bilayer properties. Fluorescent phospholipids are widely used in liposome formulation, lipid nanoparticle development, membrane fusion studies, endocytosis research, and in vivo imaging of lipid-mediated transport processes.
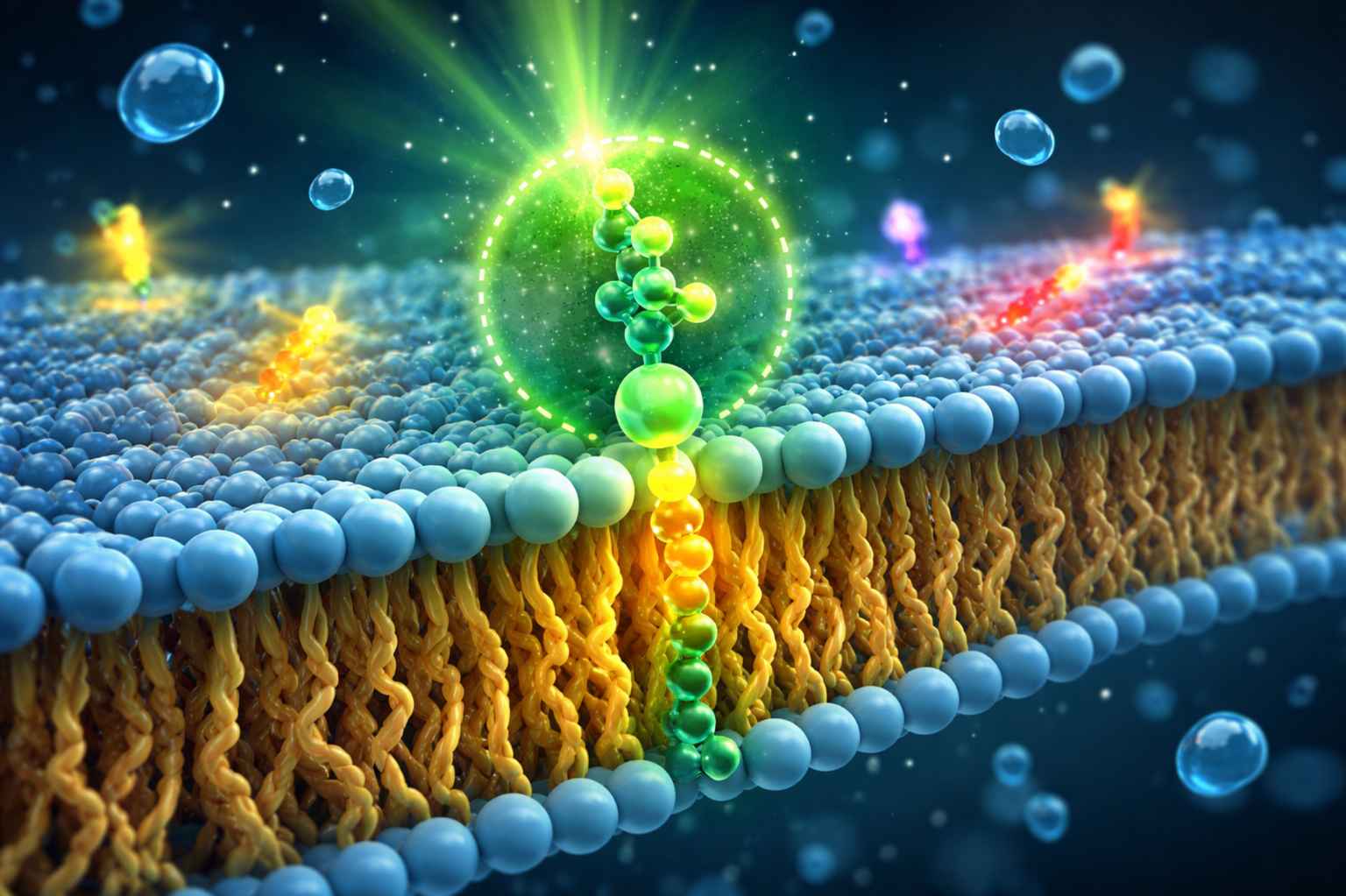 Illustration of a fluorescent phospholipid embedded in a lipid bilayer, highlighting fluorophore placement for membrane dynamics, liposome tracking, and lipid nanoparticle research.
Illustration of a fluorescent phospholipid embedded in a lipid bilayer, highlighting fluorophore placement for membrane dynamics, liposome tracking, and lipid nanoparticle research.What Problems We Solve
We design labeling strategies that preserve native phospholipid packing, phase behavior, and bilayer stability, minimizing artifacts in membrane studies.
Carefully selected lipid-compatible fluorophores and optimized attachment sites ensure stable signal retention during imaging, formulation, and storage.
Controlled synthesis and purification deliver uniform dye incorporation, enabling reproducible liposome and nanoparticle formulation.
Our fluorescent phospholipids are designed to reflect clinically relevant lipid compositions used in drug delivery and nanomedicine platforms.
Our Fluorescent Phospholipid Services
We provide specialized fluorescent phospholipid services designed to support enterprise-level research in nanomedicine, drug delivery, membrane biology, and translational imaging. Our services cover commonly used phospholipid classes and clinically relevant lipid formats, with precise control over fluorophore positioning, lipid composition, and analytical quality. Each project is supported by validated lipid chemistry, scalable purification workflows, and application-driven technical guidance.
 Fluorescent Phosphatidylcholine (PC) & Phosphatidylethanolamine (PE)
Fluorescent Phosphatidylcholine (PC) & Phosphatidylethanolamine (PE)
Capabilities include:
- Headgroup-labeled and acyl chain–labeled PC and PE phospholipids
- Fluorescent PC/PE compatible with liposomes and lipid nanoparticles
- BODIPY-, NBD-, and DiI-based lipid probes
- Controlled dye positioning to preserve bilayer packing and fluidity
- High-purity lipid preparation via chromatographic purification
- Suitable for membrane dynamics, fusion, and uptake studies
Common applications:
Liposome tracking, membrane fluidity analysis, LNP formulation development
 Fluorescent Phosphatidylserine (PS) & Phosphatidylglycerol (PG)
Fluorescent Phosphatidylserine (PS) & Phosphatidylglycerol (PG)
Capabilities include:
- Fluorescent PS and PG for charge-dependent membrane studies
- Labeling strategies preserving native headgroup charge
- Compatibility with apoptotic cell recognition and immune assays
- Stable membrane insertion for cellular uptake studies
- Analytical validation for formulation reproducibility
- Custom lipid ratios for biologically relevant membranes
Common applications:
Cell–membrane interactions, immune recognition, lipid trafficking
 PEGylated Fluorescent Phospholipids
PEGylated Fluorescent Phospholipids
Capabilities include:
- Fluorescent PEG-lipids for lipid nanoparticles and stealth liposomes
- Controlled PEG chain lengths for steric accessibility
- Fluorophore placement optimized for surface visualization
- Compatibility with clinically relevant LNP compositions
- Reduced fluorescence quenching in complex formulations
- Scalable preparation for formulation screening and optimization
Common applications:
LNP biodistribution, surface ligand tracking, in vivo imaging
 Custom Fluorescent Phospholipid Design & Supply
Custom Fluorescent Phospholipid Design & Supply
Capabilities include:
- Custom phospholipid headgroups, acyl chains, and fluorophores
- Site-specific labeling using validated lipid chemistry
- Selection of fluorophores based on imaging modality
- Batch-to-batch consistency for enterprise R&D programs
- Analytical documentation supporting regulated research workflows
- Technical consultation throughout project execution
Common applications:
Drug delivery development, translational imaging, membrane research
Fluorophores Used for Phospholipid Labeling
Fluorophore selection for phospholipids requires balancing brightness, photostability, and lipid compatibility. We focus on dyes validated for membrane insertion, minimal self-quenching, and compatibility with advanced imaging systems used in pharmaceutical and translational research.
| Fluorophore | Excitation / Emission (nm) | Compatible Lipid Systems | Primary Applications | Key Properties |
| BODIPY FL | 503 / 512 | Phospholipid bilayers, liposomes, LNPs | Membrane dynamics, lipid diffusion | Compact size, minimal membrane perturbation |
| NBD | 466 / 539 | Headgroup- or acyl-labeled phospholipids | Lipid metabolism, enzymatic assays | Environment-sensitive fluorescence |
| DiI | 549 / 565 | Liposomes, cellular membranes | Membrane labeling, vesicle fusion | Strong hydrophobic membrane insertion |
| DiD | 644 / 665 | Lipid nanoparticles, in vivo lipid systems | Biodistribution, in vivo imaging | Near-IR emission, low autofluorescence |
| ATTO 647N | 644 / 669 | PEGylated phospholipids, surface-labeled lipids | High-resolution and super-resolution imaging | Exceptional photostability, sharp emission |
Our Labeling Chemistry & Methods
Fluorescent phospholipid labeling requires precise control over fluorophore placement to preserve native lipid behavior. Our platform integrates well-established lipid modification strategies, enabling controlled incorporation of fluorophores into phospholipid headgroups or acyl chains. Each method is selected based on lipid class, intended application, and compatibility with downstream formulation or imaging workflows.
| Labeling Strategy | Lipid Modification Approach | Applicable Phospholipid Types | Technical Advantages |
| Headgroup Labeling | Covalent fluorophore attachment to polar headgroups | PE, PS, PG | Preserves hydrophobic core and bilayer integrity |
| Acyl Chain Labeling | Fluorophore incorporation into fatty acyl chains | PC, PE, PS | Stable membrane insertion and diffusion tracking |
| PEG-Linker Integration | Fluorophore conjugation via PEGylated phospholipids | PEG-PE, PEG-DSPE | Improved steric accessibility for surface visualization |
| Click Chemistry | Azide–alkyne cycloaddition on functionalized lipids | Custom-modified phospholipids | High specificity, mild reaction conditions |
Quality Control & Data Delivered
All fluorescent phospholipids are validated using analytical techniques standard to lipid chemistry and pharmaceutical development, ensuring structural integrity, fluorescence performance, and formulation compatibility.
| QC Item | Description / Method | Delivered Data |
| Purity Analysis | HPLC or TLC | Chromatograms, purity percentage |
| Structural Confirmation | LC-MS | Molecular weight and identity report |
| Fluorescence Characterization | Excitation and emission spectroscopy | Spectral profiles |
| Incorporation Efficiency | Formulation-based evaluation | Incorporation metrics |
| Stability Assessment | Storage and photostability testing | Stability and handling report |
General Workflow for Fluorescent Phospholipid Services
We assess your target phospholipid class (e.g., PC, PE, PS, PG, cardiolipin), intended use (liposome/LNP formulation, membrane biophysics, cellular trafficking, or in vivo studies), and preferred imaging platform. Based on these inputs, we recommend fluorophore families and labeling positions (headgroup, acyl chain, or PEG-linked) to balance detectability with membrane fidelity.
We confirm lipid identity and starting material format (powder or solution), review solvent compatibility, and define handling conditions to reduce oxidation and hydrolysis. If you provide a lipid blend or formulation target, we align specifications (molar ratios, PEG content, charge lipids) to ensure the fluorescent phospholipid integrates as intended.
Fluorescent phospholipids are prepared using lipid-appropriate modification strategies selected for the lipid class and fluorophore chemistry. We control labeling position and dye loading to minimize self-quenching and avoid disrupting bilayer behavior, supporting reproducible performance in liposomes, LNPs, and model membranes.
Products are purified to remove unreacted dye and side products using lipid-suitable purification workflows (e.g., chromatographic or preparative methods appropriate for hydrophobic amphiphiles). The goal is high-purity fluorescent phospholipid material that performs consistently when incorporated into lipid bilayers and formulated particles.
Each fluorescent phospholipid is verified for identity and purity by analytical methods standard to lipid chemistry (e.g., LC-MS and chromatographic purity checks) and characterized for fluorescence performance (excitation/emission profile). Where needed, we can review incorporation behavior in representative lipid systems to support formulation development.
You receive the fluorescent phospholipid with documentation for handling and storage (light protection, temperature guidance) and practical suggestions for incorporation into liposomes or LNPs (mole percent ranges and mixing considerations). Our technical team supports follow-up questions on imaging setup, assay design, and troubleshooting to help ensure reliable downstream results.
Advantages of Our Fluorescent Phospholipid Services
Our fluorescent phospholipid designs prioritize native bilayer behavior by carefully controlling fluorophore placement and lipid composition. This ensures that membrane fluidity, phase behavior, and lipid–lipid interactions remain representative of physiological and formulation-relevant systems.
Our fluorescent phospholipids are designed to integrate seamlessly into lipid compositions commonly used in pharmaceutical research, including liposomes, PEGylated lipids, and lipid nanoparticles. This ensures translational relevance from early research through preclinical development.
We emphasize controlled synthesis, analytical validation, and batch-to-batch consistency to support enterprise-scale research programs. This reproducibility is critical for comparative studies, formulation optimization, and collaborative R&D environments.

A range of membrane-compatible fluorophores and phospholipid classes can be combined to match specific imaging modalities, detection platforms, and experimental goals, allowing tailored solutions without compromising lipid functionality.
Each fluorescent phospholipid is supported by analytical data confirming purity, structure, and fluorescence performance, providing transparency and confidence for regulated and collaborative research environments.
Our team provides technical guidance on fluorophore selection, lipid formulation, and experimental design, helping research teams integrate fluorescent phospholipids effectively into complex workflows.
Applications of Fluorescent Phospholipids
Nanomedicine & Drug Delivery
- Track lipid nanoparticle and liposome distribution in vitro and in vivo.
- Evaluate formulation stability, lipid mixing, and particle integrity.
- Support optimization of delivery systems for nucleic acids and small molecules.
- Enable comparative studies across lipid compositions and formulation conditions.
Membrane Biology & Biophysics
- Study lipid bilayer organization, phase separation, and membrane fluidity.
- Investigate lipid diffusion, exchange, and membrane remodeling processes.
- Visualize lipid trafficking in cellular and model membrane systems.
- Support quantitative analysis of membrane-associated events.
In Vivo Imaging & Biodistribution
- Monitor lipid biodistribution and clearance in animal models.
- Support non-invasive imaging of lipid-based delivery systems.
- Compare targeting efficiency across formulations.
- Generate data to inform translational and preclinical studies.
Analytical & Assay Development
- Develop fluorescence-based assays for lipid–protein interactions.
- Quantify lipid incorporation and stability in complex formulations.
- Support high-throughput screening of lipid compositions.
- Enable standardized comparison across research programs.
What Our Research Clients Say

"The fluorescent phospholipids were well characterized and integrated smoothly into our liposome formulations. Clear documentation and consistent performance helped us accelerate membrane dynamics studies without extensive re-optimization."
— Dr. L., Senior Scientist, Pharmaceutical Formulation Group (Europe)
"Their guidance on fluorophore placement and lipid composition was particularly valuable for our lipid nanoparticle work. The fluorescent phospholipids enabled reliable tracking of particle behavior across multiple experimental runs."
— Ms. R., Principal Research Associate, Global Biotechnology Company
"We used the fluorescent phospholipids for in vivo biodistribution studies and observed stable signal performance with good batch-to-batch consistency. This supported comparative evaluation of several LNP formulations."
— Dr. K., Lead Investigator, Asia-Pacific Nanomedicine Research Institute
Custom Fluorescent Phospholipid Solutions for Advanced Research and Development
Whether you are developing lipid nanoparticles for nucleic acid delivery, optimizing liposome formulations, or investigating membrane dynamics and lipid trafficking, we provide custom fluorescent phospholipids designed to meet enterprise research requirements. Our team works closely with formulation scientists, membrane biologists, and translational researchers to define phospholipid composition, fluorophore placement, and purity specifications that align with your experimental and regulatory needs. From early-stage feasibility studies to large-scale, reproducible supply, we deliver fluorescent phospholipid solutions supported by rigorous analytical validation and technical consultation. Contact us to discuss your project requirements, request technical guidance, or obtain a tailored quotation.
Frequently Asked Questions (FAQ)
Fluorescent phospholipids are used to visualize and quantify lipid behavior in biological membranes and lipid-based delivery systems. Typical applications include membrane dynamics studies, liposome and lipid nanoparticle tracking, lipid trafficking in cells, and biodistribution analysis in preclinical research.
When designed correctly, fluorescent phospholipids have minimal impact on membrane structure. Fluorophore type, labeling position, and lipid concentration are carefully controlled to preserve bilayer fluidity, phase behavior, and lipid–lipid interactions relevant to physiological and formulation conditions.
Common fluorophores include BODIPY, NBD, DiI, DiD, and near-infrared dyes. These fluorophores are widely used because they are compatible with lipid bilayers, exhibit sufficient photostability, and support imaging techniques such as confocal microscopy, flow cytometry, and in vivo fluorescence imaging.
Yes. Fluorescent phospholipids are frequently incorporated into lipid nanoparticles to study particle formation, lipid distribution, cellular uptake, and biodistribution. PEGylated fluorescent phospholipids are especially useful for monitoring nanoparticle surface exposure and circulation behavior.
Fluorescent phospholipids labeled with long-wavelength or near-infrared fluorophores are suitable for in vivo imaging. These dyes reduce tissue autofluorescence and allow improved signal-to-noise ratios in animal models used for pharmacokinetic and biodistribution studies.

