Protein-PNA Conjugation
In recent years, protein-DNA conjugates have been widely used to model self-assembling protein complexes in biological systems, such as assembly of enzymatic networks, highly ordered protein arrays, biomolecular delivery systems, etc. However, the high negative charge density of DNA can limit its practical application as a scaffold in self-assembled protein systems. As a DNA mimic, PNA has better stability than DNA. Unlike the DNA backbone, the skeleton of PNA can be altered by replacing glycine with different amino acids, such as lysine or cysteine, to provide active amine or thiol groups that allow PNA to bind specifically to proteins. Considering that PNA can efficiently conjugate to proteins through expressed protein ligations with high specificity, the self-assembled fluorescent protein system based on PNA conjugates has demonstrated a novel approach to controlling protein assembly. The unique structure and characteristics of PNA and the ability to study current model systems with fluorescence measurements make it a promising system for studying protein assembly under controlled conditions.
With our experts' expertise in click chemistry and years of experience in organic synthesis, BOC Sciences is able to offer coupling protein-PNA conjugation services that are unmatched in the industry.
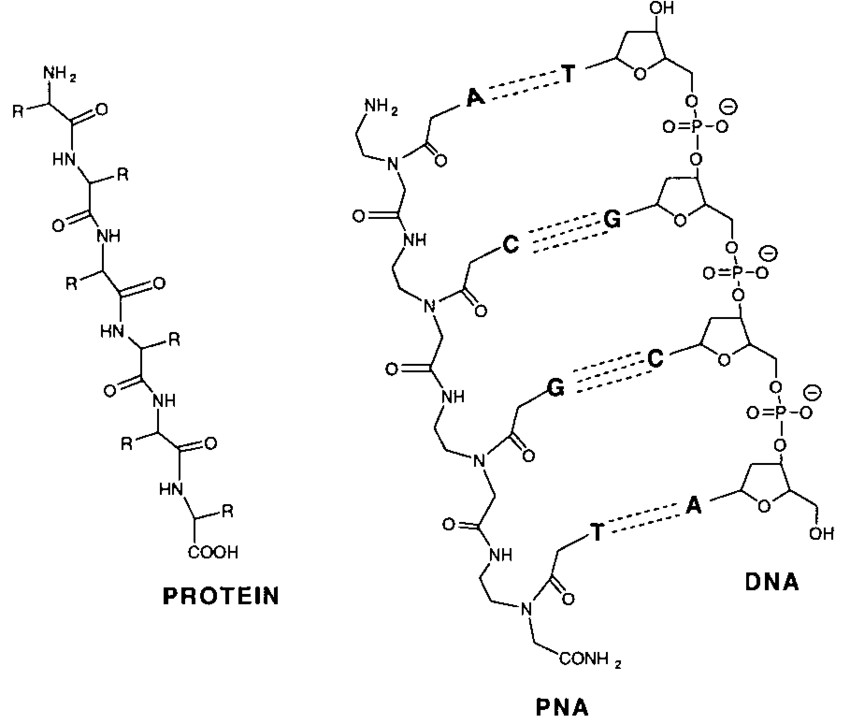 Fig 1. Structural representations of protein, PNA, and DNA. (Tyler, B. M.; et al. 1999)
Fig 1. Structural representations of protein, PNA, and DNA. (Tyler, B. M.; et al. 1999)
Application of Protein-PNA Conjugates
Protein-PNA conjugates hold great promise for the study of DNA-protein interactions, as well as being versatile molecular tools for biotechnology and materials science.
- The specificity of nucleic acid base pairing is exploited for the fabrication of protein arrays or other supramolecular devices
- Some immunological bioassays are designed and developed based on the application of these protein-PNA conjugates
- The precise recognition properties of of PNA for complementary oligonucleotides allows for directed assembly of dimers and higher oligonucleotides
- Protein-PNA conjugates have a clear photophysical signature, and therefore can be a practical tool for studying protein aggregation behavior in biochemical and cellular environments
- In the presence of single-stranded binding (SSB) proteins, one strand of PNA can efficiently invade double-stranded DNA in a sequence-specific manner, protein-PNA conjugates therefore have great potential for a number of in vivo applications
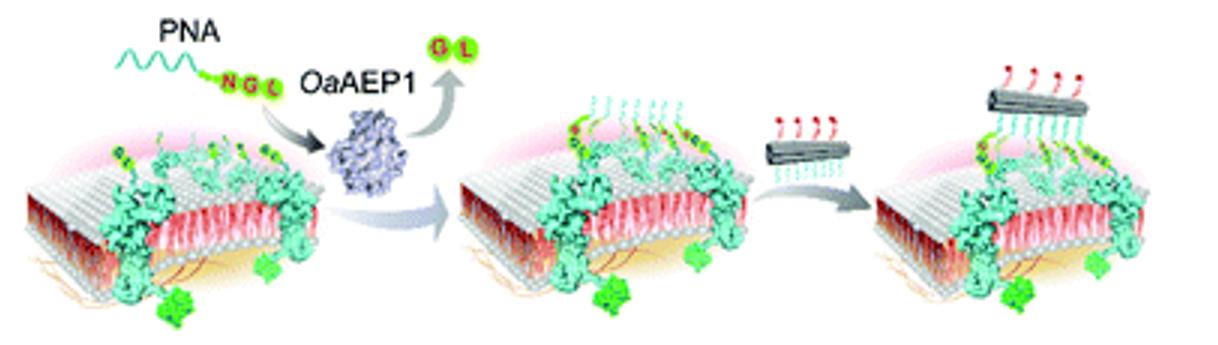 Fig 2. OaAEP1-mediated PNA-protein conjugation. (Zhangwei, L.; et al. 2021)
Fig 2. OaAEP1-mediated PNA-protein conjugation. (Zhangwei, L.; et al. 2021)
Our Services
Biologically stable PNA chains can be used as a versatile tool for nucleic acid hybridization, and protein ligases can efficiently and site-specifically conjugate proteins to PNAs. At BOC Sciences, we employ strict expressed protein ligation (EPL) to covalently ligate proteins to peptide nucleic acids. This fast ligation is achieved by joining a short N-terminal GL dipeptide in the target protein to a C-terminal NGL tripeptide in the PNA.
Protein-PNA Ligation
- An excess of PNA is added to the mTFP solution and the solution is incubated for 60 min at room temperature. Site-selected mTFP-peptide nucleic acid (mTFP-PNA) conjugates are achieved by covalently linking PNAs to proteins via expressed protein ligation
- Finally, products are analyzed by MALDI-TOF MS, and yields are quantified by UV-Vis spectrophotometry
Advantages of Our Protein-PNA Conjugation Services
- The method we developed is flexible and versatile for a variety of application
- BOC Sciences supports different types of proteins conjugated to PNA chains
Frequently Asked Questions (FAQ)
Protein conjugation can significantly enhance the targeting capabilities of PNAs. By linking specific proteins, the conjugate can be directed toward particular cellular markers or regions, improving the precision with which PNAs interact with their intended genetic targets.
The protein component of the conjugate serves to shield the PNA from rapid degradation, ensuring that it remains intact in challenging biological conditions. This added protection increases the stability of PNAs, making them more effective for long-term experiments and analysis.
Proteins can enhance the solubility of PNAs, which are typically hydrophobic and prone to aggregation. By conjugating the PNA to a soluble protein, the conjugate becomes more dispersible in aqueous solutions, facilitating better performance in biological studies.
Proteins can be selected based on their affinity for specific cellular receptors or genetic sequences, allowing the PNA to be more selectively targeted to certain cells or genetic regions. This specificity improves the accuracy and efficiency of gene manipulation processes.


References
- Tyler, B. M.; et al. Peptide nucleic acids targeted to the neurotensin receptor and administered i.p. cross the blood-brain barrier and specifically reduce gene expression. Proceedings of the National Academy of Sciences of the United States of America. 1999. 96(12): 7053-7058.
- Zhangwei, L.; et al. OaAEP1-mediated PNA-protein conjugation enables erasable imaging of membrane protein. Chemical Communication. 2021. 60.