Oligonucleotides Conjugated with Small Molecules
BOC Sciences has established comprehensive oligonucleotide conjugation platform covering the oligonucleotides modified with a various of small molecules. We commitment to advancing oligonucleotide small molecule conjugates research, and we strive to unlock the full spectrum of possibilities that these conjugates offer.
Oligonucleotide Small Molecule Conjugates
In the realm of modern drug development, the integration of oligonucleotide therapeutics and small molecules has emerged as a promising strategy. Oligonucleotide small molecule conjugates combining the oligonucleotides with the benefits of small molecules. Oligonucleotide-based therapeutics, such as antisense oligonucleotides (ASO) and siRNA, demonstrate considerable potential in controlled environments; however, their successful application in vivo is hindered by several challenges. These challenges primarily pertain to issues of biodistribution, metabolic stability, and potential toxicity. For example, oligonucleotides are susceptible to enzymatic degradation by exo- and endonucleases, resulting in abbreviated half-lives within living organisms. Additionally, their inherent polyanionic structure hampers efficient uptake by target cells or tissues. Erosion in extracellular compartments like blood and tissues, intracellular breakdown within the reticuloendothelial system (RES), and rapid renal excretion collectively curtail their therapeutic efficacy in vivo.
To surmount these obstacles, the utilization of oligonucleotide-small molecule conjugates emerges as a promising strategy to enhance selectivity and targeting, thus facilitating direct delivery of therapeutic oligonucleotides to disease-associated sites. This approach concurrently diminishes off-target effects.
The oligonucleotide modifications encompasses four distinct categories:
a. Nucleobase modifications
b. Modifications of internucleoside linkages
c. Alterations or substitutions of the ribose unit
d. Exploration of alternative backbone architectures.
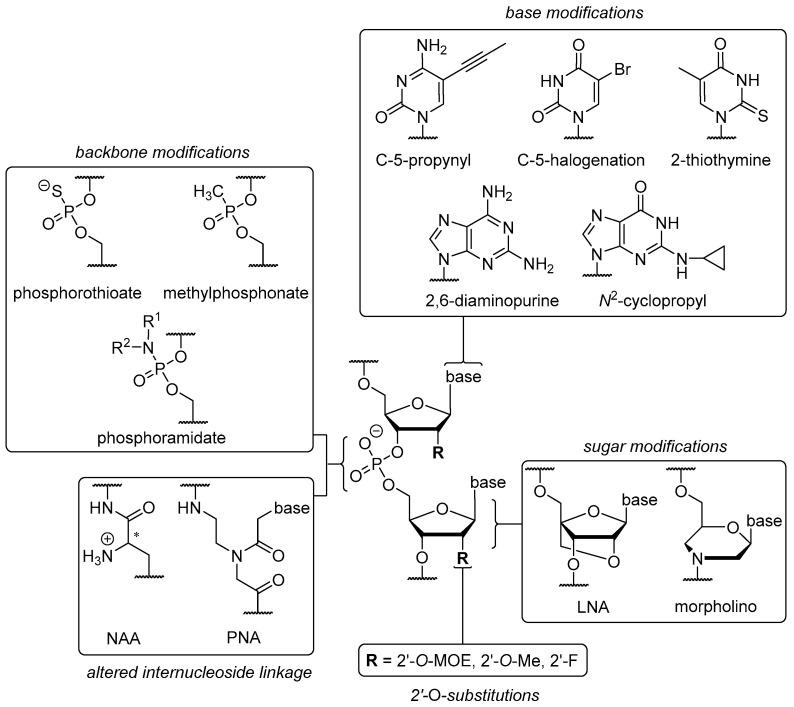 Figure 1. Available structural modification sites of oligonucleotide to small molecule. (Hawner, 2020)
Figure 1. Available structural modification sites of oligonucleotide to small molecule. (Hawner, 2020)
Oligonucleotide Small Molecule Conjugation Services
- Chemotherapeutic Small Molecule-Oligonucleotide Conjugates
- Antisense Oligonucleotide-Small Molecule Conjugates
- siRNA-Small Molecule Conjugates
- Small Molecule Ligand-Oligonucleotide Conjugates
- Lipophilic Small Molecule-Oligonucleotide Conjugates
- Peptide-Oligonucleotide Conjugates
- Aptamer-Small Molecule Conjugates
- Photoactivatable Small Molecule-Oligonucleotide Conjugates
- Peptide Nucleic Acid (PNA)-Small Molecule Conjugates
- Fluorescent Small Molecule-Oligonucleotide Conjugates
- Anandamide-siRNA Conjugates
- Folate-siRNA Conjugates
- Carbachol-siRNA Conjugates
- GalNAc-siRNA Conjugates
- Cholesterol-siRNA Conjugates
- α-Tocopherol-siRNA Conjugates
- Squalene-siRNA Conjugates
- Fatty Acid-ASO Conjugates
Design of Oligonucleotide Small Molecule Conjugates
The design of oligonucleotide small molecule conjugates includes selecting small molecules, designing oligonucleotides, and choosing appropriate conjugation methods, optimizing spacer, and considering delivery vehicle, in order to ensure effective binding, stability, and cellular uptake.
Conjugation of Small Molecule Ligands to Oligonucleotides
Combining small molecule targeting moieties (receptor ligands) with oligonucleotides presents a compelling strategy for addressing the challenges of limited cellular uptake and non-specificity associated with oligonucleotides. When devising such systems, the selection of appropriate ligand-receptor combinations necessitates careful consideration of the following criteria:
(i) Ensuring a strong binding affinity between the ligand and the specific receptor.
(ii) Evaluating variations in receptor expression patterns among the intended cell types.
(iii) Assessing the abundance of the targeted receptor.
(iv) Understanding the receptor's dynamic trafficking between the plasma membrane and endosomal compartments.
Conjugation of Lipophilic Small Molecules to Oligonucleotides
The penetration of biological membranes by highly charged polar oligonucleotide sequences is naturally hindered. This limits the overall activity of oligonucleotides in vivo due to hampered cellular uptake and limited release from endocytotic vesicles. Cellular delivery can be improved by attaching lipophilic moieties to oligonucleotide to exploit endogenous lipoprotein transport machinery. Therefore, it can be considered to design lipid-oligonucleotide conjugates to promote cellular uptake and endosomal release.
How to Conjugate Oligonucleotide to Small Molecule
- Chemical Conjugation: Covalent linkages are established between functional groups on the small molecule and the oligonucleotide using reactive chemical agents.
- Click Chemistry: Highly specific reactions, such as azide-alkyne cycloaddition, facilitate rapid and selective conjugation.
- Hybridization: Non-covalent conjugation is achieved through complementary base pairing between a modified oligonucleotide and a small molecule-tagged counterpart.
- Enzymatic Ligation: Enzymes catalyze the conjugation reaction between a small molecule and an enzymatically compatible oligonucleotide sequence.
Frequently Asked Questions (FAQ)
Small molecules can modify the solubility, stability, and hybridization efficiency of oligonucleotides. These modifications are useful for improving binding affinity and enhancing experimental outcomes.
Common methods include chemical reactions such as amide bond formation, click chemistry, and disulfide exchange, which allow precise control over the conjugation process.
Small molecules can increase or decrease hybridization efficiency based on their size, charge, and structure. This can optimize oligonucleotide performance in detection or amplification assays.
Linkers ensure that small molecules are properly positioned to interact with targets while preventing interference with the oligonucleotide's function. The type of linker can impact solubility and stability.


Reference
- Hawner, M., and Ducho, C., Cellular Targeting of Oligonucleotides by Conjugation with Small Molecules, Molecules, 2020, 25(24): 5963.