Peptide-Drug Conjugation (PDC)
As a leading CRO, BOC Sciences has been supporting customers at the forefront of drug conjugation. Peptide-drug conjugate (PDC) is an emerging promising class of targeted therapeutics. We offer peptide drug conjugation services to accelerate your peptide drug development. Our scientific team has expertise in peptide conjugation chemistry and is capable of meeting your unique project needs.
Find out more with Drug Conjugation Services.
What is Peptide Drug Conjugate?
The principle of peptide-drug conjugate (PDC) is to conjugate cell-targeting peptides with drug molecules to enhance drug targeting and to concentrate the drug in the target tissue, thus reducing its relative concentration in other tissues, increasing effectiveness and reducing adverse effects. The peptides used in PDCs include cell-targeting peptides (CTPs) and cell-penetrating peptides (CPPs). Various linkers have been used in PDCs development to prevent unspecific release of the drug. Payloads available in the PDCs include cytotoxic drugs and radionucleotides. PDC integrates the advantages of peptides with low molecular weight and biodegradability, while not causing immunogenic reactions.
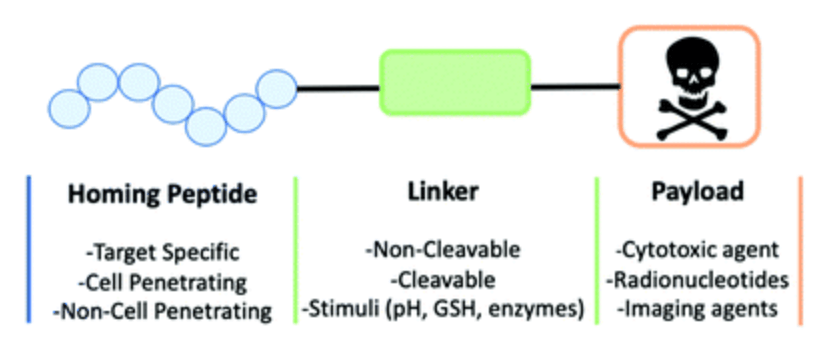 Fig 1. Peptide-drug conjugate (Cooper, 2021)
Fig 1. Peptide-drug conjugate (Cooper, 2021)
Peptide-drug conjugates (PDCs) consist of three main components, including peptide, linker, and payload.The roles and mechanisms of action of each component are important considerations during the assembly of PDCs.
Toxin drugs are integral to tumor-killing. Highly toxic drug with IC50 values in the subnanomolar range, such as maytansine, camptothecin derivatives, auristatin, Gemcitabine, paclitaxel, Zoloft Melphalan, radionuclides (177Lu-dotatate), or doxorubicin, are recommended in the PDC conjugates. The conjugated cytotoxins meet the followingfour conditions: a clear mechanism of action, small molecular weight, high cytotoxicity, and retained anti-tumor activity after chemical coupling to the peptide.
Peptides selected for PDCs should have the ability to specifically target protein receptors overexpressed in tumor tissue and have strong binding affinity for the target site within nanomolar amounts. Most of the peptides reported so far are linear peptides showing good binding. In addition, the binding affinity of peptides can be increased by stabilizing secondary structures such as α-helix and β-sheet.
| Peptide | Receptor |
| RGD (arginine-glycine-aspartic acid) | Integrins (α5β1, α8β1 and αIIbβ3) |
| GnRH (gonadotropin-releasing hormone) | GnRH-R (receptor version of the hormone) |
| SST (somatostatin) | SSTR1-5 (somatostatin receptor) |
| EGF (epidermal growth factor) | EGFR: HER1, HER2, HER3, HER4 |
| Angiopep-2 | LRP-1 (low-density lipoprotein receptor-related protein-1) |
Table 1. Examples of peptides used in PDCs
The common functional groups found in the linker region can be broadly divided into four groups: enzyme cleavable (ester, amide, and carbamate), acid cleavable (hydrazone, acetal, ketal, and carbonate), reducible disulfide, and noncleavable (thioether, oxime, triazole, and succinimidyl thioether).
1.Enzyme cleavable linker
(1) Ester and amide
Three molecules of paclitaxel are covalently linked to a single peptide (Angiopep-2) via the side chain of two lysine residues and the N-terminus amine of the peptide, resulting in the formation of the peptide−drug conjugate ANG1005. ANG1005 has showed significant activity against CNS tumors, leading to improved symptoms and an overall increase survival. This effect is particularly notable in LC patients with HER2-positive and HER2-negative breast cancers.
A conjugate of a radionuclide with peptide octreotide, called lutetium 177 DOTA-TATE, was FDA approved in 2018 for the treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEPNETs).
(2) Carbamate
Carbamate linkers employed in the PDCs undergo cleavage within intracellular endosomes or lysosomes under enzymatic conditions. Several somatostatin receptor (SSTR) targeting conjugates using carbamate linker. Among them, the most effective conjugate JF-10-81 showed potent antitumor and antiangiogenic activity.
(3) Dipeptide or Tripeptide
Specific dipeptide or tripeptide sequences can further promote the release of the active drug from the PDCs. PDCs with linkers containing dipeptide (Val-Cit or VC) can be selectively cleaved by enzymes like carboxypeptidase and cathepsin B. The dipeptide is conjugated to Dox via a para-aminobenzyl carbamate (PABC) spacer, which undergoes spontaneous 1,6-elimination subsequent to the enzymatic cleavage of the C-terminal amide of citrulline.
Another peptide sequence utilized for specifically release of drugs at the tumor site is the Ala-Ala-Asn (AAN) tripeptide, which is cleaved by enzyme legumain.
(4) Amide and a Noncleavable Thioether (Succinimidyl thioether)
(5) Ester and a Noncleavable Triazole
An azide−alkyne cycloaddition to form a triazole has been applied to link peptide with a drug in PDCs due to its ease and efficiency.
2. Acid cleavable linker
(1) Hydrazone
Hydrazone is a common acid-sensitive linker in the design of several drug conjugates. The hydrolysis and stability of hydrazone-forming ketones and hydrazines can vary depending on the original ketones from which the hydrazone is derived. For example, doxorubicin's aliphatic C-13 ketone-derived hydrazone linkers.
3. Reducible disulfide linker
Disulfide linker is used to design PDCs for targeting the EphA2 receptor that is highly expressed in several solid tumors.
4. Noncleavable linker
(1) Oxime
Several PDCs are synthesized by introducing an oxime bond to the C-13 carbonyl group of doxorubicin or daunorubicin. These conjugates are subsequently evaluated for their in vitro and in vivo properties, particularly in the context of targeted therapy for breast, colorectal, and pancreatic cancers.
(2) Triazole
The non-cleavable triazole formed through the alkyne-azide reaction imparts stability to the PDCs structure. Other bonds connecting different regions of the conjugate may facilitate drug release from the PDCs following cellular uptake. An interesting example of a conjugate with a triazole linker is a PDC developed for cancer treatment using photodynamic therapy (PDT).
| Name | TTP | Payload | Linker | Indication | Development phase |
| ANG1005 | Angiopep-2 | Paclitaxel | Succinic acid | Leptomeningeal metastases Glioma Glioblastoma brain tumor, recurrent Breast cancer brain metastases Advanced solid tumors with and without bain metastases | Phase III Phase II Phase II Phase I |
| GRN1005 | Angiopep-2 | Paclitaxel | Succinic acid | Breast cancer brain metastases; non-small cell lung cancer (nsclc) with brain metastases | Phase II |
| BT1718 | MT1-MMP binder | DM1 | Disulfide | Advanced solid tumours non-small cell lung cancer non-small cell lung sarcoma oesophageal cancer | Phase I/II |
| BT5528 | EphA2 binder | MMAE | Amide | Solid tumours EphA2-positive NSCLC | Phase I |
| BT8009 | Nectin-4 binder | MMAE | Amide | Solid tumors | Phase I |
| TH1902 | TH19P01 | Docetaxel | Succinic acid | Solid tumors | Phase I |
| TH1904 | TH19P01 | Doxorubicin | Succinic acid | Solid tumors | |
| G-202 (mipsagargin) | DgEgEgEgE | Thapsigargin | Amide | Solid tumors | Phase II |
| NGR015 (NGR-hTNF) | CNGRCG(1,5 SS) | hTNF | Amide | Malignant pleural mesothelioma | Phase III |
| tTF-NGR | GNGRAHA | tTF | Amide | Malignant solid tumors lymphomas | Phase I |
| PEN-221 | fCYwKTCC (2,7 SS) | DM-1 | Disulfide | Neuroendocrine tumors arcinoma, small cell lung | Phase I/II |
| CBP-1008 | CB-20BK | MMAE | Amide | Advanced solid tumor | Phase I |
| CBP-1018 | LDC10B | MMAE | Amide | Lung tumor | Phase I |
| SOR-C13 | folate | MMAE | Amide | Advanced malignant solid neoplasm | Phase I |
| Melflufen (delisted) | Flufenamide | Melphalan | AcOH | Multiple myeloma | Approved for marketing |
| 177Lu-dotatate (Lutathera) | Tyr-3-octreotate | 177Lu | DOTA | Neuroendocrine tumors | Approved for marketing |
| 177Lu-PSMA-617 | Glu-urea-R | 177Lu | DOTA | Prostate cancer | Phase I |
| [18F]AlF-NOTA- octreotide | octreotide | 18F | NOTA | PET or GEP-NETs; Neuroendocrine tumors | Phase I/II/III |
| [18F]Fluciclatide | RGD | 18F | PEG | PET imaging | Phase II |
| [18F]RGD-K5 | cyclo(RGDfK) | 18F | NOTA | PET imaging | Phase II |
| 68Ga-NODAGA-E[cyclo(RGDyK)]2 | E[cyclo (RGDyK)]2 | 68Ga | NODAGA | PET imaging | Phase II |
| 68Ga-NOTA-BBN-RGD | cyclo(RGDyK) and BBN | 68Ga | NOTA | PET/CT and PET imaging | Phase I |
| 90Y-DOTATOC | 3Tyr-octreotate | 90Y | DOTA | PRRT | Phase II |
| 99mTc-3PRGD2 | 3Tyr-octreotate | 99mTc | 3PRGD2 | Breast cancer SPECT/CT scan | Phase I |
| 111In-DTPA-octreotide | 3Tyr-octreotate | 111In | DTPA | Brain and central nervous system Tumors PET imaging | Phase I |
Table 2. Peptide-drug conjugates in clinical trials and approved for marketing. (Fu, Chen; et al.2022)
Although a peptide can provide many properties to PDC, including enhanced tumor penetration, reduced immunogenicity, and cheaper synthesis, the relatively small size of peptide allows for fast renal clearance of PDC. This problem can be addressed by various methods, such as chemical modification (e.g. peptide dendrimers) and physical techniques (e.g. combination of the PDC with nanoparticles).
Our Services for Peptide Drug Conjugates
- Binding affinity determination: surface plasmon resonance (SPR), biolayer interferometry (BLI) and isothermal titration calorimetry (ITC)
- Linker selection: cleavable linkers (e.g. acid cleavable, reducible disulfides, enzyme cleavable), non-cleavable linkers
- Peptide drug conjugate stability assay
- Peptide drug delivery
Related Peptide Conjugation Services
- Oligonucleotide Peptide Conjugates
- Peptide RNA Conjugates
- Antibody Peptide Conjugates
- PEG Peptide Conjugates
- Peptide Polymer Conjugates
Applications of Peptide Drug Conjugate
PDC drugs are mainly used in anti-cancer therapy. Peptide part is used for the targeted delivery of anticancer drug molecules and to reduce systemic toxicity. In some cases, PDC employs cell-penetrating peptides, which can facilitate the proper translocation of anticancer drugs across cell membranes to their desired sites.
In addition to improving pharmacological properties, PDCs can also be used for cancer diagnostics. In this case, the tumor-selective peptides are conjugated to fluorescent dyes, the peptides readily bind to specific receptors on tumor cells, and the fluorescent dyes make imaging of cancer tissue feasible.
Our Advantages
- People and suites that specialize in PDC development, manufacturing, and testing
- Various peptide-drug conjugation methods
- A wide range of characteristic analysis
- Data analysis, detailed report with results and discussion
Frequently Asked Questions (FAQ)
Peptide-drug conjugation involves attaching a peptide to a small molecule, typically to enhance the stability, solubility, or specificity of the drug. This technology is widely used in research to create targeted delivery systems, improve molecular interactions, and explore protein-ligand relationships in various biological studies.
Common conjugation methods include covalent binding through click chemistry, thiol-maleimide reactions, and amide bond formation. These methods are selected based on the desired stability, solubility, and efficiency of the conjugate, and are tailored to suit specific research needs, from protein targeting to drug solubility improvement.
By attaching drugs to peptides, the resulting conjugates often exhibit improved solubility, stability, and longer shelf life. The peptide acts as a stabilizer, preventing degradation and enhancing the pharmacokinetic profile, which is essential for studying compound efficacy and optimizing drug delivery.
The main challenges include ensuring efficient conjugation without compromising peptide activity, optimizing the drug-to-peptide ratio (DAR) for stability, and ensuring that the conjugates perform consistently under different experimental conditions.


References
- Cooper, B. M., et al. Peptides as a platform for targeted therapeutics for cancer: peptide-drug conjugates (PDCs), Chem. Soc. Rev., 2021, 50, 1480-1494.
- Alas, M., Saghaeidehkordi, A. and Kaur, K., 2020. Peptide–drug conjugates with different linkers for cancer therapy. Journal of medicinal chemistry, 64(1), pp.216-232.
- Fu, Chen, et al. "Peptide–drug conjugates (PDCs): a novel trend of research and development on targeted therapy, hype or hope?." Acta Pharmaceutica Sinica B 13.2 (2023): 498-516.