Peptide RNA Conjugation
BOC Sciences is a leading CRO specializing in drug conjugation, we provide peptide RNA conjugation services to our clients in the biotech and therapeutic areas to help accelerate your drug development programs.
Peptide RNA Conjugate
RNA peptide conjugates represent a unique amalgamation of RNA, the genetic messenger of cellular instructions, and peptides, short chains of amino acids with versatile functionalities. The peptide moiety can serve as a targeting ligand, specifically binding to certain receptors or cells. The RNA component can then be used to deliver therapeutic molecules or information, such as gene silencing. RNA peptide conjugates have been designed for RNA therapeutics, drug delivery, and screening of peptide libraries in mRNA display methods.
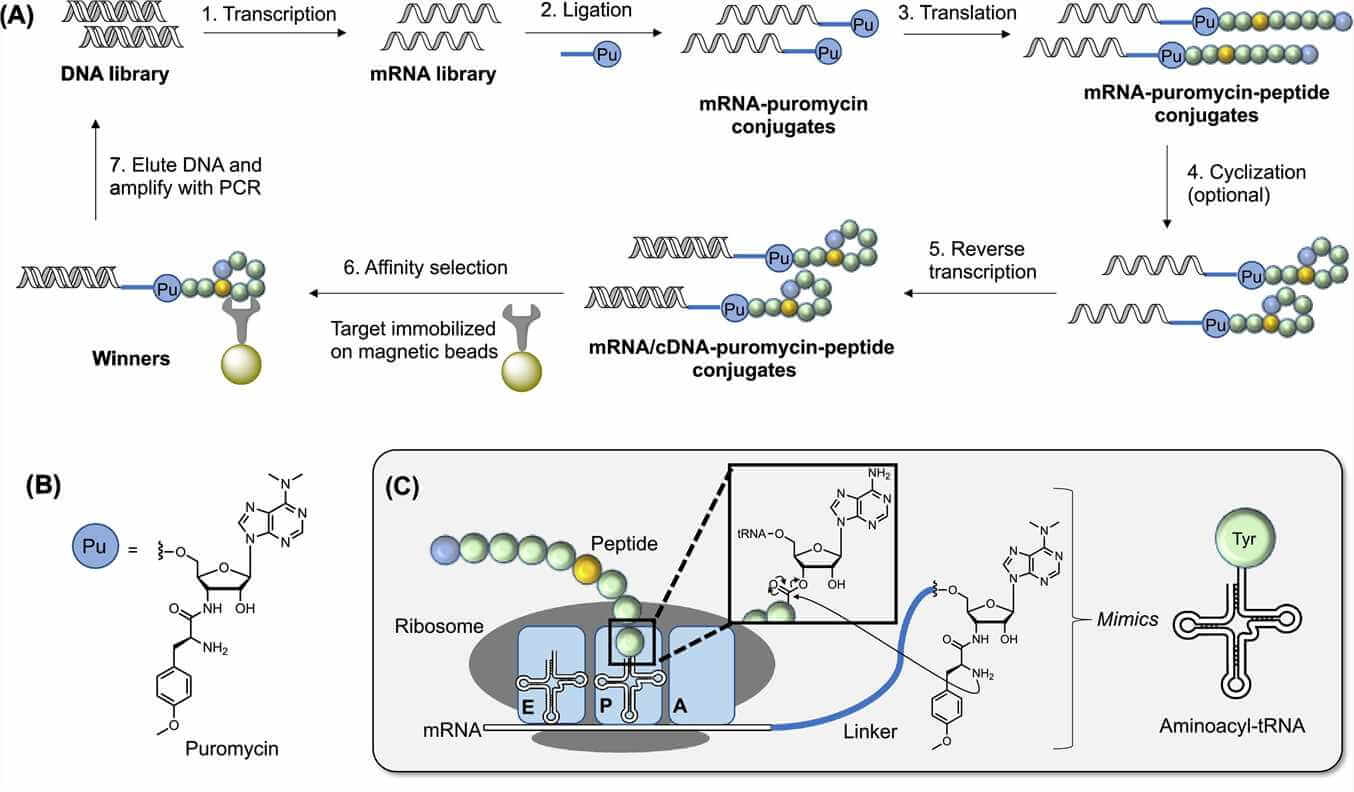 Fig 1. Peptides-RNA conjugates in mRNA display. (Peacock. 2021)
Fig 1. Peptides-RNA conjugates in mRNA display. (Peacock. 2021)
Peptide RNA Conjugation Services
BOC Sciences excels in customizing conjugation processes to meet the unique requirements of diverse projects. This customization includes the selection of appropriate linkers, chemistries, and conjugation strategies like amidation, thiol modification, and click reaction, ensuring the creation of RNA-peptide conjugates for specific applications.
Peptide Conjugation
- Peptide oligo conjugation
- Peptide DNA conjugation
- Peptide conjugated phosphorodiamidate morpholino oligomer
RNA Peptide Conjugation Techniques
- Post-synthetic conjugation / Post-assembly conjugation / Fragment conjugation
- Total stepwise synthesis / On-line solid-phase synthesis
- Local and template-directed ligation
Peptides in RNA Delivery
Peptides, with their ability to recognize specific cell surface receptors, ensure the efficient delivery of RNA cargo. Cell-penetrating peptides (CPPs) have emerged as powerful tools for RNA delivery for membrane-impermeable nucleic acids to cells and tissues. Some CPPs are derived from human, mouse, and viral biomolecules. CPP is internalized by cells via direct cell membrane penetration or endocytosis. CPPs, including cationic, amphipathic, and hydrophobic peptides, can be flexibly designed and can be easily synthesized by reliable methods such as solid-phase peptide synthesis. In addition, cationic CPP can also interact with anionic biomacromolecules (such as RNA) through electrostatic interactions. These properties make CPP suitable for use as an RNA delivery vehicle.
RNA Oligonucleotide Synthesis
Creating effective RNA peptide conjugates begins with RNA oligonucleotide synthesis. RNA sequences need to be precisely designed to ensure that the resulting RNA molecules carry the intended genetic information or regulatory function. In solid-phase synthesis, the growing RNA strand is anchored to a solid support, usually a resin. Synthesis proceeds in a stepwise fashion, with nucleotide phosphoramidites modified with protecting groups being added sequentially to the growing RNA chain, with activation and deprotection steps enabling controlled ligation.
Applications of RNA Peptide Conjugates
siRNA interference has been widely used in gene silencing, and intracellular delivery of siRNA oligomers has been the focus of research for a long time. Chemical conjugation of siRNA to cationic cell-penetrating peptide provides an promising approach to enhance antisense and siRNA delivery to cells and tissues.
By generating libraries of mRNA encoding different peptide sequences and concatenating them with their corresponding peptides. These libraries can be screened against targets of interest, allowing rapid identification of peptides with desired properties such as binding specificity or therapeutic potential.
The sensitivity of RNA and the specificity of peptides make RNA-peptide conjugates ideal candidates for biosensor development. By incorporating imaging moieties such as fluorophores into the conjugates, specific cellular processes or molecular targets can be visualized, aiding in early detection and monitoring of disease.
Our Advantages
- Customized peptide synthesis and modification
- RNA oligonucleotide synthesis and screening
- Ensure sequence integrity of RNA and peptides
- Various separation and purification techniques, such as centrifugation, electrophoresis, size exclusion chromatography (SEC) or reversed-phase HPLC
- Characterization of RNA-peptide conjugates, such as MALDI-TOF, ESI, LC-MS, fluorescence spectrometry
Frequently Asked Questions (FAQ)
Peptide-RNA conjugation significantly enhances RNA delivery by improving the molecule's ability to penetrate cell membranes. This process aids in more efficient cellular uptake, ensuring that RNA reaches its target cells effectively and with reduced degradation.
Peptide-RNA conjugation serves as a powerful strategy for molecular biology due to its ability to enhance RNA functionality. The process allows for RNA to be modified for better tissue penetration, stability, and controlled release, making it useful across a variety of research fields, including gene therapy, RNA vaccine development, and RNA editing.
The conjugation process boosts RNA's ability to overcome biological barriers, such as cell membranes and tissue layers, which are typically challenging for RNA-based therapies. The attached peptide acts as a facilitator, enhancing RNA's interaction with lipid-rich environments and enabling more efficient cellular uptake, allowing RNA to reach target cells, tissues, and organs that would otherwise be difficult to access.


Reference
- Peacock, H. and Suga, H., Discovery of De Novo Macrocyclic Peptides by Messenger RNA Display, Trends in Pharmacological Sciences, 2021, 42(5), 385-397.