Cholesterol-Conjugated Peptide
The development of peptide has focused on the conjugation of peptides to pharmacokinetics (PK)-enhancing moieties. One of the most common approaches to improve PK properties is to facilitate the binding of peptide drugs to plasma proteins by conjugation of peptide lead compounds with cholesterol. The main purpose of cross-linking the lipid moiety of cholesterol to a peptide is to increase the hydrophobic properties of the latter and its lipid-solubility, so that the conjugate could cross the highly lipophilic cell membrane and enter the cytoplasm.
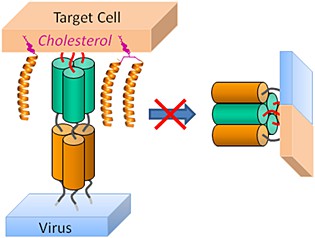 Fig 1. Cholesterol-conjugated peptide antivirals. (Pms, A. 2015)
Fig 1. Cholesterol-conjugated peptide antivirals. (Pms, A. 2015)
Application of Cholesterol-conjugated Peptide
Cholesterol-conjugated peptide for gene delivery
The properties in gene delivery of different cholesterol-peptide conjugates have been evaluated in vitro. The results have shown they can cause higher gene expression efficiency.
Cholesterol-conjugated peptide antivirals
For all enveloped viruses, fusion between the viral and the target cell membrane is a mandatory step of the life cycle. Therefore, interfering with this process has become a reliable therapeutic strategy. Viral fusion is mediated by specialized proteins that can often be used to identify the sequences of candidate peptide inhibitors directly from genomic information to develop specific antiviral drugs. If the peptide lead derived from genomic information are not sufficiently potent, it is necessary to modify them by peptide engineering strategies. Cholesterol plays a key role in the fusion and budding of enveloped viruses. It has been shown that cholesterol depletion from virus-infected cells can inhibit virus production by inhibiting virus-cell fusion. Therefore, it has been proposed that the addition of cholesterol groups to peptides will increase their affinity for membranes, and the resulting increase in local concentration at the site of action will translate into enhanced antiviral potency. Cholesterol-conjugated peptide antivirals are a new and powerful tool for rapid response to viral diseases. Cholesterol-conjugated peptides enable to effectively bind to cellular and viral membranes to exert their antiviral activity.
Advantages of Cholesterol-conjugated Peptide
- Improved pharmacokinetics (PK) by solving the problem of short half-life in vivo due to enzymatic degradation and rapid clearance.
- Cholesterol-conjugated peptide promotes the blood-brain barrier penetration.
- Cholesterol-conjugated peptide drugs are active in vivo.
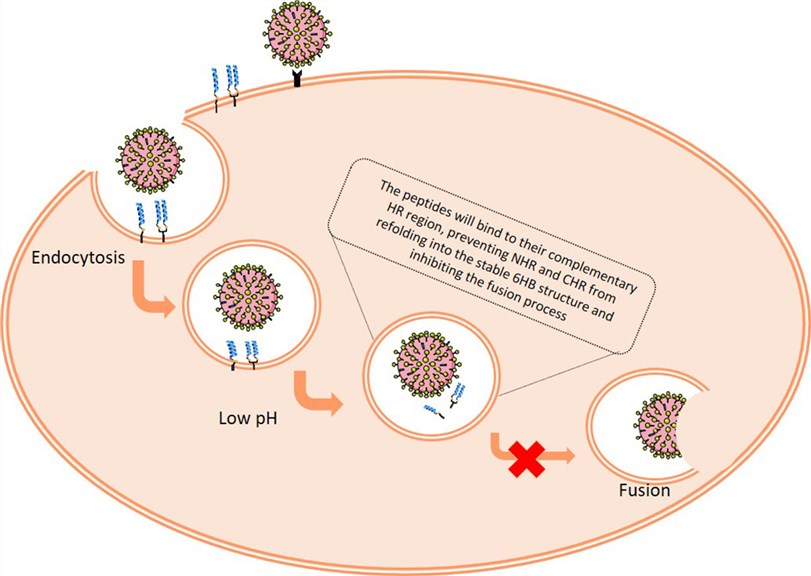 Fig 2. Cholesterol-conjugated peptide inhibitors of influenza virus. (Pms, A.; et al. 2017)
Fig 2. Cholesterol-conjugated peptide inhibitors of influenza virus. (Pms, A.; et al. 2017)
Our Services
Synthesis of Cholesterol-conjugated Peptide for Gene Delivery
Our experts offer services for the synthesis of amphiphilic cholesterol-conjugated peptide by selecting hydrophobic cholesterol and positively charged peptides. This unique cholesterol-conjugated peptide we have synthesized is able to efficiently condense DNA and can be used as a gene carrier in intravenous therapy.
Synthesis of Cholesterol-conjugated Peptide Inhibitors of Virus
BOC Sciences provides novel strategies for the synthesis of cholesterylated peptide fusion inhibitors that could contribute to the development of new antiviral drugs.
- Conjugation Methods
A standard solid-phase Fmoc protocol and a chemoselective thioether conjugation methods are applied to conjugate peptides to cholesterol.
- Synthesis Process
We can synthesize artificial peptides by performing derivatization of peptide chains with cholesterol and dimerization to complete the cholesterol conjugation. The same cysteine-containing precursor peptide used to produce the monomeric cholesterol-conjugated peptide can form a partial reaction with the cholesterol dimer-forming moiety, which makes it possible for the parallel synthesis of cholesterol-conjugated monomers and dimers. Trimers or higher-order multimers are prepared using the same strategy.
Frequently Asked Questions (FAQ)
By attaching cholesterol to peptides, their functionality is greatly enhanced, particularly in terms of membrane interaction and stability. Cholesterol incorporation allows peptides to better integrate into lipid-rich environments, thereby improving their ability to cross cell membranes, engage with specific cellular targets, and maintain stability under physiological conditions. This modification is crucial for optimizing the peptide's performance in biological processes.
Cholesterol-conjugated peptides offer improved solubility, enhanced cell membrane penetration, and more consistent delivery. These advantages make them ideal for applications such as protein interactions, gene regulation studies, and other research where cellular uptake and stability are crucial.
The effectiveness of cholesterol conjugation depends on several factors, such as the choice of linker, the peptide's structure, and the specific biological environment. Optimization of these factors ensures that the cholesterol-conjugated peptide performs effectively in the desired application.
Cholesterol can be conjugated to a wide range of peptides, provided the peptide is designed to work effectively with lipid-based conjugates. The process can be adjusted to suit different peptide lengths, sequences, and functional groups, making it versatile for various applications.


References
- Pessi, A. Cholesterol-conjugated peptide antivirals: a path to a rapid response to emerging viral diseases. Journal of Peptide Science. 2015.
- Pms, A.; et al. The pH-sensitive action of cholesterol-conjugated peptide inhibitors of influenza virus. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2021. 1863(12): 183762.