Peptide-PNA Conjugation
Though PNAs have been found to have a wide range of chemical and biological applications, PNAs may be limited by low solubility, poor intracellular delivery, and renal clearance. The conjugation of PNAs to peptides, lipophilic molecules, and cell-specific receptor ligands offers a new approach to address this issue. The covalent binding of peptides to PNAs can help overcome some of the development challenges. Cell-penetrating peptide (CPP) has been found to have strong cellular translocation properties and drug delivery potential. Currently, a variety of cell delivery studies have focused on the covalent conjugates of CPPs with various types of cargoes including oligonucleotides and their analogs. Some reported data has showed that PNAs covalently attached to CPPs enhance cell delivery and biological activity. For example, CPP conjugation can increase the solubility of PNAs and reduce its physiological clearance by promoting cellular internalization. Therefore, peptide-PNA conjugates have promising applications in diagnostic and therapeutic fields.
BOC Sciences can provide reliable services for the synthesis of peptide-PNA couples, and our experts are able to achieve high coupling efficiencies using click chemistry, allowing for the fabrication of long (>15-mers) PNA-peptide conjugates.
Application of Peptide-PNA Conjugates
PNA-CPPs conjugates have potential to be effective tools for a variety of biomedical applications, including:
- Gene editing
- Gene regulators
- Antibacterial agents
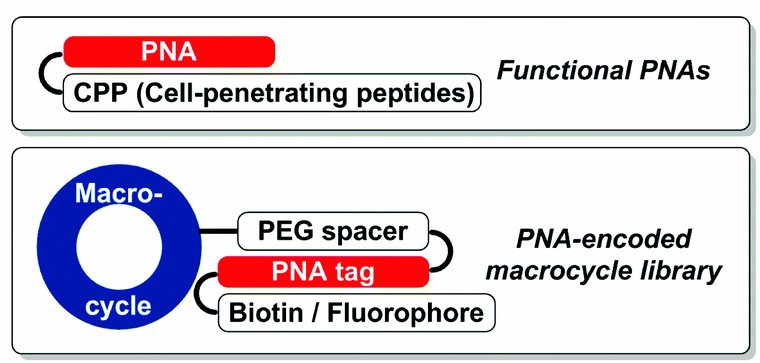 Fig 1. Applications of PNA-peptide conjugates. (Chao, Y.; et al. 2014)
Fig 1. Applications of PNA-peptide conjugates. (Chao, Y.; et al. 2014)
Our Peptide-PNA Conjugation Services
Through peptide bonds, PNAs can be easily conjugated to the target peptides since PNAs are able to recognize the corresponding complement of DNA or RNA by Watson-Crick base-pairing. Due to the correct intra-molecular spacing and hybridization between complementary nucleic acids, peptide nucleic acids show high sequence selectivity and affinity towards DNA or RNA molecules of the peptide. At BOC Sciences, we have used a Boc-protection strategy to synthesize multiple PNA-peptide conjugates in parallel. In our method, the selected peptides are ligated to the N-terminus of the PNA. The data results of HPLC/MALDI-TOF analysis has approved that our approach is able to yield products with 90% purity, suitable for screening of large numbers of PNA and/or peptide sequences for biochemical and biological studies.
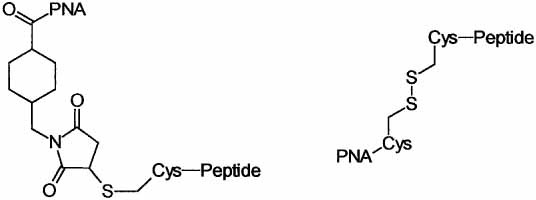 Fig 2. PNA-Peptide Conjugates. (Awasthi, S. K.; Nielsen, P. E. 2002)
Fig 2. PNA-Peptide Conjugates. (Awasthi, S. K.; Nielsen, P. E. 2002)
Deprotection and Purification of PNAs
Analysis and purification of PNAs is performed by using the reversed-phase high performance liquid chromatography (HPLC)
Peptide Synthesis
We synthesize C-terminal amide peptides via the standard Fmoc solid-phase synthesis techniques, and the products are purified by reversed-phase HPLC and MALDI-TOF mass spectrometry with the same matrix as for PNA
Synthesis of CPP-PNA conjugates
A series of stably-linked CPP-PNA conjugates are synthesized by the Fmoc method, in which the linkage between the PNA and peptide fractions consisted of a short polyether linkage. All conjugates are purified by reversed-phase HPLC and characterized by MALDI-TOF mass spectrometry. In the CPP-PNA conjugates, a single CPP can be attached to the N-terminal or C-terminal end of the PNA.
Why Choose BOC Sciences?
- Certificate of Analysis (COA) with detailed quality control documentation
- RP-HPLC trace and Mass Spec analysis
- Performed and supervised by applying various state-of-the-art instruments
Frequently Asked Questions (FAQ)
The conjugation of peptides to PNAs can significantly increase the binding affinity of the PNA for specific nucleic acid sequences. Peptides can be engineered to target particular cellular receptors, improving PNA's ability to engage with the intended genetic targets for precise research applications.
Unlike traditional nucleic acid delivery methods, peptide-PNA conjugates do not rely on viral vectors. They offer a more controllable and potentially safer alternative for delivering PNAs to specific cellular targets, with the added benefit of reducing immune responses commonly associated with viral delivery systems.
Peptides serve as functional agents that enhance the solubility, membrane permeability, and tissue penetration of PNAs. By carefully choosing peptides with optimal properties, the conjugates become more effective in reaching and interacting with specific genetic targets, improving the overall functionality of the PNA.


References
- Chao, Y.; et al. Facile solid-phase synthesis of PNA-peptide conjugates using pNZ-protected PNA monomers. Organic Chemistry Frontiers. 2014. 1. 1050-1054.
- Awasthi, S. K.; Nielsen, P. E. Synthesis of PNA-Peptide Conjugates. Methods in Molecular Biology. 2002. 208(3): 43.