Enzyme Labeling
Enzyme labeling is a high precision technique that involves attaching enzymes to molecules such as antibodies or antigens and using them as markers. It is significant for revealing presence, location and quantifying specific biological molecules in research specimens. It serves as an essential tool to detect and measure biological events occurring at a cellular and molecular level. As a professional biotech company, we offer enzyme labeling services with the highest standards.
What is Enzyme Labeling?
Enzyme labeling is an immunolabeling technique that covalently binds enzyme molecules to antibodies or antigens. This conjugation does not alter the immunoreactivity of antibodies or antigens nor affect the biochemical activity of enzymes. When corresponding substrates are added, substrates are catalyzed by enzymes to reveal complementary reaction color, and color depth is proportional to the content of corresponding antigens or antibodies. Enzyme-labeled substances include enzyme-labeled antigen, enzyme-labeled antibody and enzyme-labeled SPA. The quality of enzyme-labeled antibodies or antigens primarily depends on purity, activity and affinity, and different enzyme labeling methods can lead to different outcomes. Nevertheless, enzyme labeling strategy is directly related to the success of Immunase technology.
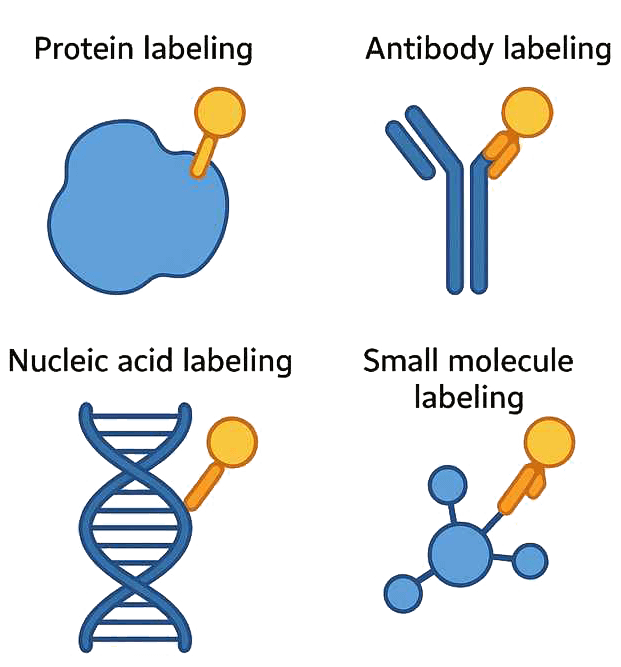
 Fig.1 Site-specific modification of proteins via enzymatic reaction
Fig.1 Site-specific modification of proteins via enzymatic reactionWhat are Commonly Used Enzymes for Labeling?
Enzymes are commonly used as 1) detectors to directly or indirectly examine protein levels in conjugation with antibodies; 2) nucleic acid hybridization probes required for sensitive assays and related methods.
It is used in immunoblotting to conjugate secondary antibodies due to its ability to oxidize substrates precisely.
AP is commonly used in immunoassays due to its high thermal stability, long-life span, and exceptional endurance to chemical denaturation.
This is often used in glucose biosensing, frequently in medical devices for the monitoring of blood glucose levels.
They are typically used in labeling due to their remarkably high binding affinity. Streptavidin and avidin are regularly employed for this purpose.
This enzyme can catalyze the hydrolysis of specific colorless substrates into colorful products, making it useful in labeling studies.
An enzyme that catalyzes bioluminescent reactions, primarily used in in-vivo imaging and reporter assays.
It is a marker enzyme, widely used in antibiotic resistance studies. It works by cleaving the β-lactam ring in antibiotics such as penicillin, thereby deactivating them.
They add phosphate groups to proteins, commonly used in labeling proteins for traceability in signal transduction pathways.
They transfer acetyl groups from one molecule to another. They're used in histone labeling, to trace epigenetic modifications.
This enzyme synthesizes DNA from an RNA template, a process used in cDNA labeling, allowing for gene expression profiling.
Why Need Enzyme Labeling?
Enzyme labeling techniques often utilize enzyme immunoassays which are highly sensitive. This is because the enzymes used in the labeling process can generate a large amount of products that can be easily detected.
Enzyme labeling techniques provide both quantitative and qualitative analysis. They can provide quantitative information about the amount of target molecule and qualitative information about the presence or absence of a particular molecule.
Enzyme labeling is highly specific. It can specifically bind to the target molecule due to the specificity of antigen-antibody interaction. This specificity greatly increases the accuracy of the testing results.
Enzyme labels are available for a wide variety of analytes and can be used in a variety of assay formats. They can be used in solution phase assays, solid phase assays, homogeneous assays, heterogeneous assays, etc.
Enzyme labeling techniques are faster than other labeling techniques, which speeds up testing processes and improves efficiency.
Enzyme labeling techniques can be applied to a number of different areas including clinical diagnostics, food and environmental testing, drug discovery and development, forensic science, etc.
Enzyme labeling techniques are cost effective. They do not require expensive detection equipment. Simple colorimetric detection method can be used for detecting enzyme labels.
Our Enzyme Labeling Services
Precision conjugation of enzymes to antibodies for enhanced detection and signal amplification in immunoassays.
Site-specific enzyme conjugation to oligonucleotides for use in nucleic acid detection and amplification workflows.
Custom enzyme-peptide conjugation to support targeted delivery, detection, or enzymatic activity tracking.
Tailored enzyme-protein conjugation to facilitate biochemical assays, signal transduction studies, or therapeutic development.
Functional enzyme attachment to nanoparticles to enable biofunctionalization, biosensing, or targeted biocatalysis.
Covalent enzyme-nucleic acid conjugation to improve assay performance in diagnostics, amplification, and molecular biology applications.
From Inquiry to Completion: Our Service Flow
1Prepare Enzymes
Choose an enzyme with high activity, good stability, easy to measure, and does not react strongly with the substance being tested.
2Synthetic Enzyme Label
The enzyme is combined with an antibody or antigen, and the usually used method is chemical coupling.

3 Enzyme Labeling Experiment
After the enzyme has been successfully conjugated, the labeled molecule can be introduced into the desired biological system. The enzyme serves as a kind of flag that allows researchers to easily locate or track the labeled molecule.
4Detection
Enzymes catalyze the conversion of substrates to produce measurable substances, usually color change or fluorescence, which can be measured by spectrophotometer or fluorescence detector.
5Result Analysis
Based on the measured signal intensity, the concentration of the target substance can be calculated.
In this process, the choice of enzyme and the quality of the enzyme label have a very important impact on the accuracy of the results. Enzymes with large amounts, good stability, and easy detection are usually selected as labeling enzymes. At the same time, the quality of the enzyme label is also very important, and it needs to have high affinity and specificity.
Advantages of Working with Us
We have extensive experience in custom PEGylation and comprehensive expertise in chemical and biological preparations.
We offer custom synthesis services and modify the properties of molecules according to customer-specific requirements.
Our proven processes and efficient workflow ensure rapid turnaround times without sacrificing quality or accuracy.
We adhere to stringent quality regulations and standards to ensure utmost product consistency, potency, and purity.
We fully respect our clients' intellectual property, maintaining strict confidentiality and offering transparent, clear communication throughout the entirety of each project.
Despite our high-quality services, we offer competitive pricing models that prove cost-effective for our customers.
We pride ourselves on our exceptional customer service, where we maintain constant communication and provide progress updates throughout each project.
Many top-tier biopharmaceutical companies around the world have chosen to partner with us, reflecting our reliability and professional expertise in PEGylation technology.
Where Our Enzyme Labeling Are Used?
Enzyme labeling is widely used in diagnostic testing. For example, in enzyme-linked immunosorbent assay (ELISA), an antibody attached to an enzyme can be used to detect the presence of a specific protein or antigen. When the antigen is present, the enzyme reacts to a substrate, producing a color change that can be measured.
Enzyme labeling is used in targeted cancer treatment. Enzymes can be attached to antibodies that can specifically recognize cancer cells. This label helps direct the antibody to cancer cells and kills them without harming healthy cells.
Enzyme labeling is essential in drug discovery processes. The method is used to evaluate the effect of potential drugs by altering the enzymes' functions on target cells.
Enzymes can be attached to drugs to aid in their delivery to specific tissues or cells. The enzyme works as a 'label' to direct the drug to the correct location in the body, enhancing the drug's effectiveness and reducing side effects.
Enzyme labeling is used in genetic research for tagging specific DNA sequences. It enables scientists to locate and identify genes of interest.
Enzyme labeling is used in the food industry for quality control and identifying pathogenic organisms. Enzymes attached to antibodies can identify specific bacteria or pathogens present in food samples.
Enzyme labeling is used in environmental monitoring to detect and measure levels of specific pollutants or toxins. The presence of these substances can trigger a reaction from the enzyme, which can be measured as an indication of pollution levels.
Enzyme-labeled compounds are used in medical imaging for the diagnosis of diseases. They allow doctors to non-invasively view the inside of the body to detect abnormalities such as tumors.
Case Study
Case Study 1
DNA damage plays a significant role in gene regulation and disease progression. Accurately quantifying DNA damage is vital for identifying potential disease biomarkers for risk assessment, early diagnosis, and treatment monitoring. However, challenges exist such as the low abundance, random location, diversity, and the difficulty in selectively amplifying DNA damage. This research proposes a method combining enzymatic labeling with single-molecule detection for the sensitive quantification of DNA damage. This approach only labels DNA sequences containing damaged bases with biotin-conjugated deoxynucleotide triphosphate (biotin-dNTP), improving detection specificity. Further sensitivity is achieved with terminal deoxynucleotidyl transferase-induced polymerization of multiple Alexa Fluor 488-labeled-deoxyuridine triphosphates and the incorporation of single-molecule detection. This method can detect DNA damage as low as 1.1 × 10−16 M and discern low-abundant DNA damage down to 1.3 × 10−4%. It can also provide data on DNA damage occurrence within a specific gene and determine DNA damage levels in various cancer cells, presenting a novel approach for researching the physiological function of DNA damage in human diseases.
 Fig. 2 Schematic illustration of the integration of enzymatic labeling with single-molecule detection for sensitive quantification of various DNA damages. (Zhang, Y., 2020)
Fig. 2 Schematic illustration of the integration of enzymatic labeling with single-molecule detection for sensitive quantification of various DNA damages. (Zhang, Y., 2020)
Case Study 2
Immunoassays rely on antibodies and labelled markers to detect target macromolecules in complex samples like serum or saliva. They are essential techniques for clinical diagnostics and research within pharmaceutical and environmental areas due to the high specificity of antibody-antigen interactions. Integrating magnetic beads (MBs) into immunoassays enhances the efficiency of target capture and allows convenient separation. Moreover, preconcentration of the target and attaching multiple enzyme labels to the MBs can significantly amplify signals, permitting detection limits to be reached that would be unachievable with traditional methods. To ensure high assay performance, a robust bead-probe conjugation protocol must be applied and bioconjugated products thoroughly characterized. In this study, MBs was functionalized with antibodies and enzyme lables for ultra-sensitive detection of protein analytes. Detection antibodies (Ab2) and horseradish peroxidase (HRP) labels attachment onto MBs, and purification of the bead bioconjugates (MB-Ab2-HRP), was facilitated by magnetic separations. When integrated with microfluidic systems, position of the resulting bioconjugate beads can be magnetically controlled to facilitate faster assay times. Heavily labeled detection particles have led to detection limits in low fg/mL range, enhancing sensitivity 100-1000 times as opposed to conventional immunoassays using singly labeled Ab2's.
 Fig.3 (A) The covalent attachment of antibodies to the tosyl functionalized magnetic particles. The tosyl groups act as leaving groups for surface amine groups present on antibodies for covalent attachment. (B) The complete conjugation protocol for both the attachment of antibodies as well as HRP enzyme labels. (Otieno, B. A., 2016)
Fig.3 (A) The covalent attachment of antibodies to the tosyl functionalized magnetic particles. The tosyl groups act as leaving groups for surface amine groups present on antibodies for covalent attachment. (B) The complete conjugation protocol for both the attachment of antibodies as well as HRP enzyme labels. (Otieno, B. A., 2016)
Frequently Asked Questions (FAQ)
We offer custom enzyme labeling services based on your investigational needs. Firstly, we review your project objectives, then our skilled scientists design and implement the most suitable enzyme labeling strategy. Final product is assessed for quality, stability and performance before delivery.
The duration of the enzyme labeling process can vary depending on the complexity and specific requirements of your project. However, we strive to deliver quality services within the discussed timeframe.
Our enzyme-labeled products are packaged and delivered under strict conditions to maintain their stability. Upon receipt, they should be stored at the temperature recommended on the product details provided. Most of our products require cold storage, typically at -20°C.
Absolutely. Our company adheres to the highest standards in bioscience research. We have rigorous quality control procedures in place to ensure the reliability, reproducibility and accuracy of our enzyme labeling services.
We use a variety of enzymes for labeling including, but not limited to, horseradish peroxidase (HRP), alkaline phosphatase (AP), and beta-galactosidase. The choice of enzyme depends on your specific requirements, the nature of your sample, and the intended downstream applications.
The cost of our enzyme labeling services depends on several factors such as the type of enzyme required, the complexity of the project, and the turnaround time. We recommend getting in touch with us for a detailed and customized quote.
Learn More About Enzyme Labeling
References
- Zhang, Y., et al. Integration of enzymatic labeling with single-molecule detection for sensitive quantification of diverse DNA damages. Analytical Chemistry. 2020, 92(7): 4700-4706.
- Otieno, B. A., et al. Bioconjugation of antibodies and enzyme labels onto magnetic beads. Methods in Enzymology. 2016, 571: 135-150.