Oligonucleotide Liposome Conjugation
With over two decades of experience, BOC Sciences has established liposome conjugation platform for designing and synthesizing liposome-oligonucleotide conjugates tailored to researchers' exact requirements.
Liposome Oligonucleotide Conjugates
Liposome oligonucleotide conjugates stand at the forefront of a new era in pharmaceuticals. These conjugates are a result of merging liposomes, versatile lipid-based vesicles, with oligonucleotides, like antisense oligonucleotides (ASOs), short DNA or RNA molecules that hold the key to manipulating genes and influencing cellular processes. The amalgamation of these two entities yields a powerful platform for the targeted delivery of therapeutic cargo.
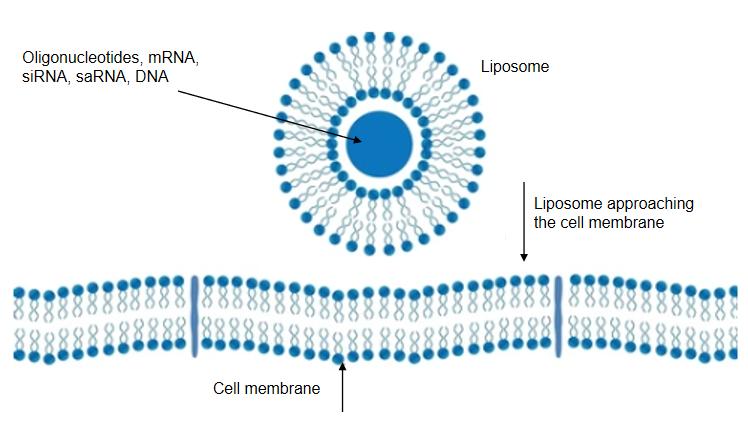
The encapsulation of oligonucleotides within liposomes is a strategy that capitalizes on the protective capabilities of these nanoscale carriers. Furthermore, the modular nature of liposomes allows for fine-tuning their properties to match the demands of a specific oligonucleotide. Liposome composition, size, and surface properties can be precisely engineered to optimize their interaction with target cells, ensuring that liposome-oligonucleotide conjugates are versatile vehicles that accommodate a wide array of therapeutic goals.
In the drug discovery, tailoring drug delivery systems to specific needs becomes essential. This is precisely where Oligonucleotide Liposome Conjugation Service Portfolios offered by BOC Sciences come into play.
Oligonucleotide Liposome Conjugation Services
- Tailored liposome composition, size, and surface properties.
- Modifying oligonucleotides for specific sequences and functionalities.
- High-efficiency encapsulation of oligonucleotides within liposomes.
- Attachment of ligands, antibodies, or peptides to liposomes for targeted interactions.
- Physicochemical characterization of liposome-oligonucleotide conjugates.
- Evaluating in vitro cellular uptake and intracellular distribution of conjugates.
- Enhancing cellular uptake and intracellular delivery.
- Determining controlled release kinetics of encapsulated cargo oligonucleotides.
- Providing expert guidance and consultation on formulation design.
- Tailoring surface modifications for specific cell targeting.
- Evaluating stability under physiological conditions.
- Creating lipid-oligonucleotide hybrid molecules.
- Implementing state-of-the-art analytical methods for thorough characterization.
What are Liposomes?
Liposomes are nanoscale lipid-based vesicles that mimic cell membranes in structure. Comprising lipid bilayers with an aqueous core, liposomes have the ability to encapsulate hydrophilic substances like oligonucleotides and drugs. This encapsulation offers protection against degradation and acts as a vehicle for delivering fragile payloads to target cells.
Types of Liposome:
- Small Unilamellar Vesicles (SUVs)
- Large Unilamellar Vesicles (LUVs)
- Multilamellar Vesicles (MLVs)
- Cationic Liposomes
- Anionic Liposomes
- PEGylated Liposomes
- pH-Sensitive Liposomes
- Thermosensitive Liposomes
- Stable Liposomes
- Stealth Liposomes
- Fusogenic Liposomes
- Hybrid Liposomes
- Antibody-Targeted Liposomes
- Long-Circulating Liposomes
Liposomes in Oligonucleotide Delivery
Oligonucleotides, due to their size and nature, face barriers that hinder their successful cellular uptake. Liposomes provide an ingenious solution by encapsulating these delicate molecules, shielding them from enzymatic degradation, and facilitating their entry into cells.
Liposome RNA Delivery
Ribonucleic acid (RNA) molecules play a pivotal role in genetic regulation and protein synthesis, making them attractive targets for therapeutic interventions. Liposomes, with their biocompatibility and customizable properties, serve as efficient vehicles for delivering RNA molecules precisely to the cells where they are needed.
Liposome mRNA Delivery
This technique harnesses the versatility of liposomes to encapsulate and deliver messenger RNA (mRNA) molecules into target cells, thereby enabling the production of specific proteins within the cell. Liposome mRNA delivery has been widely used in mRNA vaccine development, cancer immunotherapy, protein replacement therapy, and tissue regeneration.
Liposome siRNA Delivery
Small interfering RNA (siRNA) presents a remarkable avenue for gene silencing, holding great potential for treating genetic disorders and diseases. Yet, delivering siRNA molecules to their intended targets within the body poses a challenge. Liposomes provide a versatile solution by encapsulating siRNA and facilitating its delivery to specific cells.
Advantages of Cationic Liposomes for RNA Delivery
Cationic liposomes carry a positive charge on their surface due to the presence of cationic lipids. This positive charge enables strong electrostatic interactions with the negatively charged RNA molecules, including mRNA, siRNA, and miRNA, facilitating their encapsulation within the liposomal structure. The lipid bilayer of cationic liposomes acts as a protective shield for encapsulated RNA molecules, preventing RNA from enzymatic degradation and other external factors that could compromise its integrity. In addition, cationic liposomes can interact with the negatively charged cell membranes through electrostatic interactions, increasing the internalization of the RNA cargo by target cells.
Oligonucleotide Encapsulation in Liposomes
- Passive Encapsulation during Liposome Formation
- Reverse Phase Evaporation
- Post-Formation Encapsulation
- Dehydration-Rehydration Vesicles (DRV) Method
- Active Encapsulation Methods
- Sonication
Frequently Asked Questions (FAQ)
Liposomes provide a nanoscale confined environment that can influence how oligonucleotide chains fold or form secondary structures. This allows researchers to investigate folding pathways and structural dynamics that differ from free oligonucleotides in solution.
OLC allows visualization of nanoscale clustering, transient domain formation, or self-assembly patterns that are difficult to capture in bulk solution, giving direct insights into molecular organization.
By anchoring oligonucleotides to liposomes, researchers can study how nucleic acids influence or respond to membrane properties, including curvature, surface potential, and local ordering effects.

