Biotinylation
Biotinylation is a process of covalently attaching biotin to proteins, peptides, antibodies, nucleic acids (DNA), and polysaccharides. This technique is useful for the purpose of labelling, purifying or detecting molecules of interest. As a professional biotech company, we provide top-notch biotinylation services, enabling the bioresearch community to unlock vast potential in exploring cellular functions, disease mechanisms, and therapeutic applications.
What is Biotin?
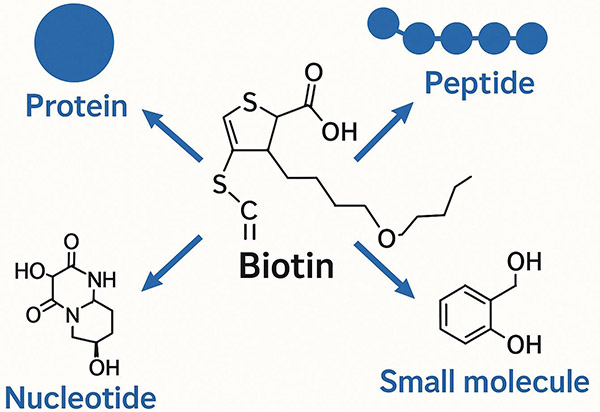
Biotin, also known as vitamin B7, vitamin H, or coenzyme R, has a molecular weight of 244.31 and is water-soluble. It is a coenzyme of carboxylase related to the synthesis of fatty acids, isoleucine, etc. Biotin is a heterocyclic compound that contains an imidazolone ring for carboxyl groups transportation. Its side chain, the carboxyl group, can be linked to the lysine residue of carboxylase through an amide bond. Furthermore, the conjugation of biotin and avidin is one of the strongest non-covalent interactions known in nature. This particular conjugation has a dissociation constant (Kd) of about 10-14 mol/L and has well toleration to various conditions (temperature, pH, solvent, etc.). Therefore, biotin and avidin are often combined to form the Biotin-Avidin System (BAS). In biological experiments, it is often necessary to label biotin to proteins or other biological materials through covalent bonds, and this process is called biotinylation. Biotin is a small molecule, so its addition does not greatly alter the properties of the substance being modified. This technology is often used in biological research because biotin has an extremely high binding affinity for proteins called streptavidins and avidins, which allows for specific and sensitive detection and purification of the biotinylated substance.
Classification of Biotinylation
Depending on the type of molecule and site of attachment, biotinylation could be classified into two broad categories:
Non-site-specific Biotinylation: This method indiscriminately labels all accessible sites on a molecule. It's useful for general-purpose labeling or when site specificity is not required.
Site-specific Biotinylation: In contrast, this method is selective and labels specific sites on a molecule. This is particularly useful when avoiding interference with a molecule's function or when studying the function of specific sites.
Why need Biotinylation?
Biotin has an incredibly high affinity for streptavidin or avidin, making it a highly specific interaction. This attribute allows for the precise targeting and isolation of biotinylated molecules.
Biotin-streptavidin interaction is one of the strongest known non-covalent interactions in nature, ensuring stable and reliable results in experimental conditions.
Biotinylation can be applied to a wide variety of biomolecules, including proteins, peptides, nucleic acids, and carbohydrates, providing flexibility in experimental design.
Certain types of biotinylation are reversible, allowing for the potential recovery of the native molecule after studies or analyses.
Biotinylated molecules can be easily detected and quantified using simple, cost-effective techniques, facilitating experimental analysis.
Biotin is a naturally occurring, non-toxic vitamin, reducing potential safety concerns in using biotin-based methods.
Due to its high binding affinity and specificity, biotinylation often yields a high signal-to-noise ratio, improving data quality.
Biotin is relatively small, often not interfering with the biological activity or function of the molecule to which it is attached.
Our Biotinylation Services
We can perform bioconjugation of your proteins, antibodies, peptides, or any other biomolecules with biotin, according to your specific requirements.
Our skilled team is able to conduct site-specific biotin conjugation on your targeted positions which is crucial for maintaining the functionality of your biomolecules.
We offer services to assess the efficiency and strength of the biotin-streptavidin interactions to validate your research studies.
All our Biotinylation services come with comprehensive quality assurance processes including HPLC, Mass Spectrometry, and SDS-PAGE analyses to ensure the highest standards are met.
We also provide professional consultation and technical support to address any queries and assist you in utilizing these services effectively.
Post-biotinylation, we handle the purification processes to ensure the product is free from unreacted biotin or other impurities.
As a reputable biopharmaceutical company, we also provide a range of Biotinylation services.
Proteins conjugated with biotin for high-affinity binding in detection, purification, or immobilization applications.
Antibodies tagged with biotin to enable specific capture, detection, or assay development using streptavidin-based systems.
DNA or RNA molecules modified with biotin for use in hybridization, detection, or pull-down assays.
Synthetic peptides functionalized with biotin to facilitate binding, tracking, or affinity purification.
Individual nucleotides modified with biotin for incorporation into nucleic acids for detection and labeling purposes.
Short DNA or RNA sequences labeled with biotin to enable probe-based detection or capture in molecular biology workflows.
Glycosphingolipids modified with biotin for use in studying lipid interactions, signaling, or cell recognition.
Magnetic nanoparticles coated with biotin for targeted separation, detection, or delivery applications.
Custom synthesis of PCR primers with biotin tags for downstream purification, detection, or immobilization.
Custom synthesis or supply of biotin-conjugated amino acids for use in peptide labeling or protein engineering.
Our biotin-streptavidin conjugation service provides highly efficient and specific binding for various applications in molecular biology, including protein interactions and assays.
Biotinylated dNTPs are essential for accurate and sensitive detection in PCR, sequencing, and other molecular biology techniques.
Our custom biotinylated phosphoramidite service is designed for efficient incorporation into DNA/RNA synthesis, perfect for labeling, detection, and analysis.
Biotinylated dUTP is ideal for efficient DNA labeling and detection in a variety of molecular assays, such as fluorescence in situ hybridization (FISH).
Our biotinylated miRNA services offer a reliable solution for miRNA labeling, detection, and profiling.
Biotin aptamers provide highly specific molecular recognition, enabling sensitive detection, targeted drug delivery, and diagnostic applications in biotechnology and medical research with strong biotin-streptavidin binding versatility.
Biotinylated lipids integrate biotin functionality into membranes, facilitating biomolecular interactions, cell labeling, and advanced drug delivery systems with reliable biotin-streptavidin binding for biotechnology and nanomedicine applications.
Cell biotinylation tags live cell surfaces with biotin, enabling efficient protein tracking, receptor studies, and interaction assays through high-affinity biotin-streptavidin chemistry for biomedical research and diagnostics.
Biotin fluorescence combines biotin labeling with fluorescent probes, providing precise molecular imaging, real-time detection, and sensitive bioassays for life sciences, diagnostic research, and biotechnology development.
Biotinylated antigens enhance immune detection assays, enabling sensitive antibody binding, improved ELISA performance, and reliable vaccine research through high-affinity biotin-streptavidin interactions.
Biotinylated gold nanoparticles enable targeted biosensing, imaging, and therapeutic delivery by combining biotin's molecular recognition with gold nanomaterial properties for biomedical and nanotechnology research.
Biotin-labeled DNA enables precise nucleic acid detection, hybridization assays, and molecular diagnostics with strong biotin-streptavidin binding for biotechnology and genetic research applications.
From Inquiry to Completion: Our Service Flow
Advantages of Working with Us
Our biotinylation services are adaptable to different project needs, ensuring you get the service most suitable for your requirements.
Our dedicated customer support team is available to address your queries and provide necessary assistance throughout the project.
From monoclonal antibodies to recombinant proteins, we are capable of offering biotinylation services for a wide range of proteins with various sizes and structures.
Our team of experts employs the most advanced analytical tools to ensure precise bioconjugation, guaranteeing both the functionality and integrity of the biotinylated proteins and antibodies after the process.

We have delivered over 100 biotinylated proteins and antibodies. Due to our extensive experience, in-depth understanding of the technology, and stringent quality control, we have been able to achieve a high rate of successful outcomes for our projects.
Our implementation of modern technology in biotinylation process results in improved antigen recognition, binding ability and detection sensitivity, which ultimately enhance your research and diagnostic results.
We understand the importance and sensitive nature of our clients' data. Hence we respect and maintain strict confidentiality of all your project information.
Our services are scalable allowing us to handle projects of any size, whether it be a single test or a large scale study.
Where Our Biotinylation Are Used?
Biotinylated molecules can efficiently bind to streptavidin, a protein known for its strong affinity to biotin, allowing for an easy and efficient separation of targeted molecules from complex samples.
Biotin-streptavidin interactions also form the basis for a myriad of assays utilized for detecting and quantifying biological substances such as antibodies, proteins, and nucleic acids.
Biotinylation is widely used for labelling cell surfaces for studies related to cell migration, interaction, and adhesion.
In pharmaceutical research, biotinylation is used for targeted drug delivery systems. The biotin molecule facilitates the delivery of the drug to the desired cell, improving the effectiveness of treatment while reducing side effects.
Case Study
Case Study 1
Proximity-dependent biotin identification (BioID) is a powerful technique in in vivo identification of protein associations. In this method, a biotin ligase, which can bind to a variety of molecules, is attached to targeted proteins living cells for labeling surrounding proteins in a defined period. This study used BioID to investigate the human nuclear pore complex (NPC), a mammoth macromolecular assembly in eukaryotes that play a crucial role in cellular component trafficking by anchoring within the nuclear envelope. By applying BioID to Nup107-160 and Nup93 complexes, two essential NPC subcomplexes, a significantly different set of NPC constituents was found contingent on the positioning of these BioID-fusion proteins within the NPC. This allowed us to gain greater insights into the Nup107-160 subcomplex by evidence of direct interaction between Nup43 and Nup85 and further refine the practical labeling radius of BioID. Consequently, this study enhances our understanding of human NPC's organization and affirms that BioID offers a valuable tool for exploring large protein assemblies' constitution and organization in living cells.
 Fig.2 Biotinylation of Y-Nups in the context of the whole NPC defines a practical labeling radius. (Kim, D I, 2014)
Fig.2 Biotinylation of Y-Nups in the context of the whole NPC defines a practical labeling radius. (Kim, D I, 2014)
Case Study 2
Past research has suggested that the vitamin biotin modifies histones H3 and H4 posttranslationally through binding, a process catalyzed by holocarboxylase synthetase (HCS). Despite biotinylated histones being a rare epigenetic mark, it has often been found in abundance in repeat regions and suppressed loci, contributing to genome stability and gene regulation. Lately, researchers were unable to find any biotinylated histones, suggesting biotinylation may not be a natural histone modification but rather an experimental artifact. In this paper, streptavidin blots and western blots of enzymes and histones were used to identify the histone of cells cultured in biotin-defined medium. These findings conclusively verify that biotinylation is a naturally occurring, albeit unusual, histone modification. Less than 0.001% of human histones H3 and H4 are biotinylated, which brings into question whether their levels are sufficient to produce biological effects in vivo. By combining the data from our study with previous research and ongoing investigations, we have developed a revised working model. In this model, the connection of HCS in chromatin results in the sporadic binding of biotin to histones, serving as an indicator for HCS binding locations.
 Fig.1 Binding of radiolabeled biotin to histones. (1. Toshinobu, K., 2011)
Fig.1 Binding of radiolabeled biotin to histones. (1. Toshinobu, K., 2011)
Frequently Asked Questions (FAQ)
Biotinylation technology involves the process of covalently attaching a biotin moiety to a protein, nucleic acid, or other molecule. This allows the labeled molecule to be detected and/or purified using the high affinity binding of biotin to avidin or streptavidin.
Our team of experts utilizes state-of-the-art techniques and materials to provide accurate and efficient Biotinylation services. Our extensive experience and knowledge ensure a robust, high quality, and reliable service.
We can biotinylate a wide range of molecules including proteins, peptides, antibodies, and nucleic acids.
Yes, we can tailor our biotinylation services to suit your specific needs and requirements.
The biotinylated molecule can be detected using avidin or streptavidin conjugated to a reporter molecule such as a fluorophore or an enzyme.
Biotinylation technology has a wide range of applications in research and diagnostics. These include cell and tissue labeling, protein-protein interaction studies, protein purification, and many others.
The time frame for our Biotinylation service can vary depending on the nature and quantity of the molecule to be biotinylated. Our expert team will discuss the details with you to provide an accurate timeline.
We combine our expertise, precision, and advanced techniques to deliver high-quality Biotinylation services that are tailored to meet your specific needs. We prioritize client satisfaction and strive to provide the highest level of service.
We take every precaution to ensure that the Biotinylation process does not adversely affect the functionality of your molecule. However, the effect may vary depending on the specific molecule.
The biotin-streptavidin interaction is one of the strongest non-covalent interactions known in nature, hence, biotinylated molecules will remain stable for long periods under appropriate storage conditions.