Protein Oligonucleotide Conjugation
Nucleic acids and proteins constitute two key classes of functional biomolecules, respectively. While nucleic acids have a limited number of structural components that make up the nucleic acid, they have well-defined hydrogen bonding properties that make them uniquely programmable and easily predictable in terms of overall geometry. Proteins have a broader range of accessible structures because they have more constituent components and a wider range of interactions between these components that can influence the overall structure. As a result, proteins have a rich structural, chemical and functional diversity. Oligonucleotides are capable of influencing the biodistribution and cellular uptake or for targeting specific tissues by covalently linking various ligands, which represent an attractive possibility for advancing therapeutic applications and expanding development options. Proteins are currently commonly used as attached ligands for the preparation of therapeutic oligonucleotide conjugates. The ability to access specific protein-oligonucleotide conjugates allows access to a broader range of functional molecules.
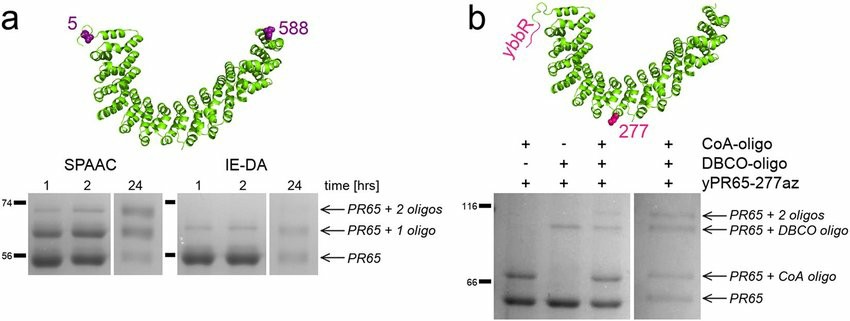 Fig 1. Attachment sites and SDS-PAGE of protein-oligo conjugations. (Synakewicz, M.; et al. 2019)
Fig 1. Attachment sites and SDS-PAGE of protein-oligo conjugations. (Synakewicz, M.; et al. 2019)
Application of Protein-Oligonucleotide Conjugates
Oligonucleotide-protein conjugates have been widely used in therapeutic and diagnostic applications such as:
- siRNA-protein delivery
- Improve the vaccine adjuvant properties of oligonucleotides
- Pre-targeted therapy of cancer
- Protein detection and quantification
Protein-Oligonucleotide Conjugation Techniques
Both non-covalent and covalent methods can be used to link synthetic oligonucleotides to proteins.
Non-covalent Strategies
The reversible non‐covalent method include biotin-streptavidin or nickel-histidine. Compared to covalent conjugation, non‐covalent method produces protein-oligonucleotide conjugates with poor stability.
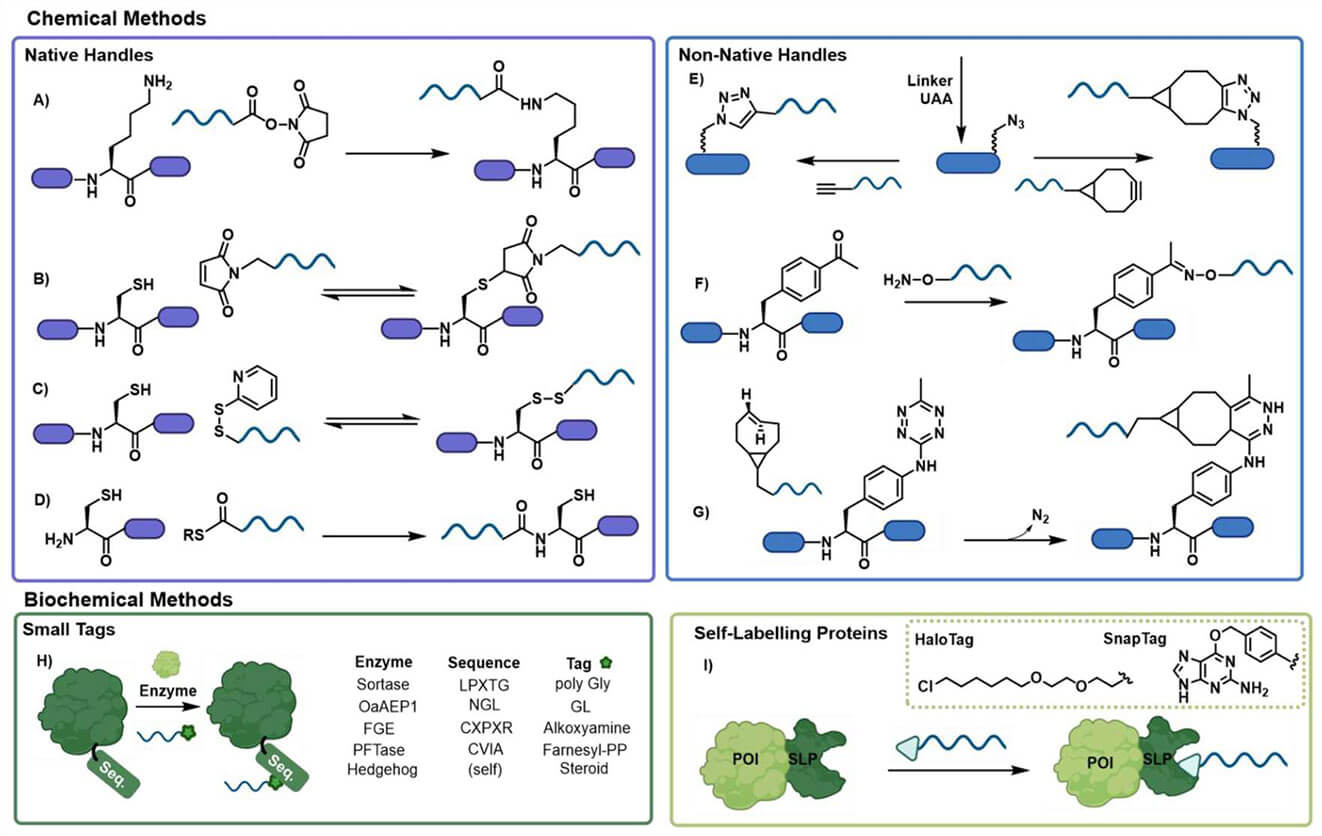 Fig 2. Conjugation chemistries utilized in the formation of protein oligonucleotide conjugates. (Watson, E. E.; Winssinger, N. 2022)
Fig 2. Conjugation chemistries utilized in the formation of protein oligonucleotide conjugates. (Watson, E. E.; Winssinger, N. 2022)
Covalent Strategies
In the covalent approach, enzymes are used to recognize small-molecule tags and transfer them to specific peptide sequences in larger protein substrates, and appropriately tagged functionalized oligonucleotides can be attached to the target protein. The covalent conjugation of some oligonucleotides to proteins is developed based on the modification of the oligonucleotide at a predetermined site, where its reactive group reacts directly with the amino group of lysine or the thiol group of cysteine to obtain an amide or disulfide bond, respectively. In addition, there are a number of oligonucleotide-protein conjugates are generated using bifunctional cross-linkers, and the most commonly employed bifunctional linkers carry maleimide and N-hydroxysuccinimide ester groups.
Our Protein-Oligonucleotide Conjugation Services
One of the most widely used strategies is conjugation through the nucleophilic amine side chain of lysine residues. This is achieved primarily by functionalizing the oligonucleotide with N-hydroxysuccinimide esters, producing a stable amide linkage following nucleophilic attack. Cysteine residues are also commonly involved in nucleophilic processing to modify proteins with maleimide functionalized oligonucleotides.
At BOC Sciences, our expert teams use the thiol-maleimide method for oligonucleotide-protein conjugation. Commonly used bifunctional cross-linkers are the heterobifunctional cross-linker succinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC), the homobifunctional cross-linker Disuccinimidyl subera (DSS), etc.
- Firstly, these bifunctional cross-linkers are coupled with the protein to produce maleimide-protein intermediates
- Then, after removal of excess cross-linker, the maleimide group is reacted with thiol-modified oligonucleotides, where the amino-modified oligonucleotides react with the bifunctional cross-linkers to produce maleimide-oligonucleotides, which eventually react with the free thiol of the protein cysteine
- Finally, ethanol and reverse-phase HPLC are used to complete precipitation and purification, respectively
Advantages of Our Conjugation Services
- Satisfactory yield: high conjugation efficiency
- Linkage stability: most stable covalent bonded conjugates
- Consistence: good reproducibility from batch to batch
- Oligo selection: both single strand oligos and double strand oligos are available
Frequently Asked Questions (FAQ)
Different proteins can influence solubility, folding, and interactions with oligonucleotides. Selecting the right protein helps achieve stable and functional conjugates for experiments.
By using site-specific conjugation sites and adjusting reaction conditions, researchers can limit the number of oligonucleotides per protein for more uniform results.
Techniques like fluorescence detection, gel electrophoresis, or simple absorbance measurements allow verification of successful conjugation and estimation of oligonucleotide loading.
Attachment sites affect the folding and accessibility of both protein and oligonucleotide. Proper placement prevents steric hindrance and ensures reproducible experimental outcomes.


References
- Synakewicz, M.; et al. Bioorthogonal protein-DNA conjugation methods for force spectroscopy. Nature Publishing Group. 2019. 1.
- Watson, E. E.; Winssinger, N. Synthesis of Protein-Oligonucleotide Conjugates. Biomolecules. 2022. 12. 1523.