Hapten-Carrier Conjugation
As a leading drug conjugation service provider, BOC Sciences is committed to offering hapten small molecule conjugation services. With advanced equipment and professional expertise, we provide comprehensive solutions for hapten-carrier conjugates.
Hapten-Carrier Conjugates
Haptens are small molecular compounds with a molecular weight below 1000 Da that cannot be effectively recognized by the immune system to induce an antibody response, such as toxins, hormones, pharmaceuticals, and pesticides. However, haptens can be chemically modified at specific sites by attaching active group-bearing linkers, and then they can be combined with carriers to create hapten-carrier conjugates, also known as artificial antigens. These conjugates can indirectly induce the proliferation and differentiation of B cells, resulting in the production of specific antibodies by utilizing T cell epitopes.
The preparation of hapten-carrier conjugates with good immunogenicity is the most critical step in establishing immunoassay methods for small molecular compounds. Immunoassays are sensitive analytical methods based on the specific recognition and reversible binding reactions between antigens and antibodies and have found extensive applications in biological analysis. Immunoassays for detecting small molecules mainly refer to indirect competitive enzyme-linked immunosorbent assay (IC-ELISA). For instance, in the case of small molecular toxin haptens, they are conjugated to carrier proteins, inducing an immune response in animals, and then tested using IC-ELISA.
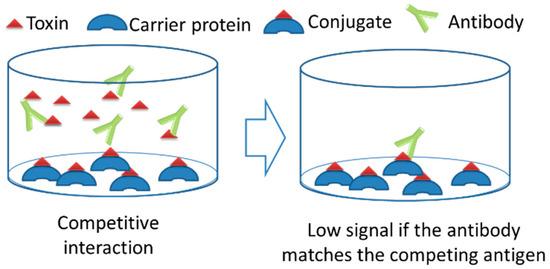 Fig. 1 Hapten protein conjugate used for IC-ELISA1
Fig. 1 Hapten protein conjugate used for IC-ELISA1
BOC Sciences provides one-stop services for hapten-carrier conjugates, including synthesis, characterization, and commercial production. We offer comprehensive support to various clients in the field of drug development.
Hapten Protein Conjugate
Proteins are commonly used carriers to induce a strong immune response. Hydrophilic carriers like bovine serum albumin, with its large reactive groups, stable properties, and solubility in aqueous or some organic solvents, are often used.
Hapten Conjugate Vaccine
When small molecule targeted drugs are not effective treatment options, immunotherapy can be used as an alternative strategy. Since small molecules are not inherently immunogenic, hapten conjugate vaccines are used to produce antibodies against these small molecule haptens, such as several abused drugs like morphine haptens and cocaine haptens.
Hapten-Labeled Nucleotides
Hapten-labeled nucleotides can be used to prepare DNA probes, such as fluorescence in situ hybridization (FISH) probe.
Examples of Hapten-carrier conjugates of clinical importance
- Penicillin is a small molecule and by itself is inherently not immunogenic. However, upon degradation in the body, it produces a highly reactive "penicilloyl" group which can react with proteins (albumin) to form a penicilloyl-protein conjugate (hapten-carrier conjugate). This is recognized as foreign and provokes an immune response. Once antibodies (IgE) are generated, they bind to IgE receptors on the surface of mast cells and basophils. If an individual in this condition is treated with penicillin, it can lead to allergic reactions that can be life threatening. Drug allergies primarily arise due to the conjugate acting as an antigen.
- Many hypersensitivity reactions are mediated by CD4 T cell, commonly referred to as delayed type of hypersensitivities. These reactions are also known as contact sensitivities, contact dermatitis or contact hypersensitivity. It is characterized by eczema appearing at the sites of contact with the allergen. Some examples include:
- Catechols found in poison ivy, poison-oak and poison sumac
- Nickel, often used in jwellery
- Some dyes
- Certain organic molecules/ chemicals used in industry. These chemicals bind spontaneously to normal proteins and so act as a hapten.
- Toxin of poison ivy (Rhus radicans), The resin of this plant, comparing a mixture of complex catechols known as urushiol, binds to proteins upon contact. This includes the skin proteins of individuals who come into contact with the plant. The skin cells modified in this way are regarded as foreign and attacked by lymphocytes in a manner similar to the rejection of a skin graft. This cell-mediated immune response leads to the formation of blisters and results in a discomforting skin rash referred to as allergic contact dermatitis.
Commonly used hapten- carrier conjugates are:
- DNP-BGG Dinitrophenol (hapten); Bovine gamma globulin (carrier)
- TNP-BSA Trinitrophenyl (hapten); Bovine serum albumin (carrier)
- ARS-OVA Azophenyl arsenate (hapten); Ovalbumin (carrier)
- LAC-HGG Phenyllactoside (hapten); Human gammaglobulin (carrier)
Coupling methods of Hapten
Hapten coupling can be achieved through various reactions, utilizing either an existing functional group or an added reactive group. Among the available coupling methods, a commonly employed procedure is the induction of amide bond formation using carbodiimides, which were initially applied to peptide synthesis. Water-soluble carbodiimides facilitate the formation of protein-hapten conjugate formation in aqueous solvents and have been extensively used for synthesizing immunogenic conjugates and to a limited extent, enzyme conjugates. The effectiveness of water-soluble carbodiimides is constrained due to the limited solubility of some haptens in aqueous solutions. Additionally, the formation of the intermediate products like O-acylisourea derivative and N-acylurea side products, which are typically less soluble than the parent molecule. Similarly, the mixed anhydride method, using an alkylchlorocarbonate reagent, can also result in conjugation of the alkylcarbonate component, that like stable acylisourea derivatives, may act as unintended haptens. Isocyanides were developed as promising reagents for the immobilization of biological molecules without compromising their activity. Researchers have adapted and modified these reactions to provide an alternative to carbodiimides in peptide synthesis. Isocyanides with tertiary amino groups, such as 2-morpholinoethylisocyanide (MEI), in the presence of a suitable additive such as N-hydroxysuccinimide (NHS), promoted peptide synthesis in high yield. Scientists previously adapted the MEI peptide synthesis procedures to prepare hapten conjugates including those associated with zeatin and benzimidazole derivatives.
 Fig. 2 Haptens conjugated to proteins using 2-morpholinoethylisocyanide
Fig. 2 Haptens conjugated to proteins using 2-morpholinoethylisocyanide
Hapten Conjugation Services
Hapten Small molecule Modification
Haptens have multiple potential modification sites. Different hapten modifications result in differences in antibody affinity, specificity, and efficacy. We synthesize various modified haptens based on different sites and choose the one that maintains the most complete hapten structure when connecting to the carrier for high affinity and strong specificity antibody production. Alternatively, we can select modifications that do not preserve the complete structure of the hapten molecule for establishing highly sensitive IC-ELISA.
Linker Selection
The linker should avoid attaching near functional groups of the target hapten and its length should be appropriate, allowing the immune system to recognize the characteristic structure of the hapten.
Conjugation
It involve using coupling agents to link haptens to carriers at functional groups like -COOH, -NH2, or -SH. We select appropriate conjugation methods based on the specific hapten being used. Additionally, we have the capability to control the molar ratio of hapten to carrier.
- Carboxyl groups: carbodiimide, mixed anhydride, and active ester
- Amino groups: glutaraldehyde, isocyanate, nitrophenyl, isourea, isocyanate chloride, and diazotization
- Hydroxyl groups: succinic anhydride, diazotized benzoic acid, and sodium chloroacetate
- Thiol groups: S-acetylthioethyl succinimide ester reaction using a disulfide bond
Hapten Carrier System
Carrier systems allow haptens to activate the immune system and generate antibodies. BOC Sciences can provide custom hapten-carrier conjugation services, selecting appropriate carrier systems to meet your needs.
- Proteins: Globulin fractions, bovine serum albumin (BSA), ovalbumin (OVA), keyhole limpet hemocyanin (KLH), rabbit serum albumin (RSA), human serum albumin (HSA), thyroglobulin, and others.
- Peptides: Synthesized polylysine, polyglutamic acid, mixed amino acid polymers, and more.
- Polymers: High-molecular-weight organic compounds such as polyvinylpyrrolidone, carboxymethyl cellulose, poly(methyl methacrylate) particles, and others.
Our Advantages
- Hapten small molecule phage display
- Hapten conjugate linker optimization
- Multiple hapten conjugation methods
- In vitro immunoassays such as ELISA and IC-ELISA
- Advanced analysis techniques such as LCMS/MS, GC/MS, Prep-Chromatography
Frequently Asked Questions (FAQ)
Hapten-Carrier Conjugation is widely used in immunology for creating vaccines, diagnostic assays, and immunological research. It is also employed in developing monoclonal antibodies and other immune-based therapies that require a targeted immune response.
A carrier protein enhances the immunogenicity of the hapten, which by itself is typically too small to elicit a strong immune response. The carrier protein acts as a scaffold, allowing the immune system to recognize and respond to the hapten more effectively.


References
- Ertekin, Ö., et al., Biological Activity of the Carrier as a Factor in Immunogen Design for Haptens, Molecules, 2018, 23(11), 2977.
- David L. Brandon & Ronald G. Binder, 2-Morpholinoethylisocyanide as a coupling agent for hapten-protein conjugates, Food and Agricultural Immunology, 17:1,53-61.
- Gupta K R, Antigens and haptens[M], 2007