Virus-like Drug Conjugation (VDC)
As a leading service provider focused on drug development, BOC Sciences has been supporting customers at the forefront of drug conjugates. Virus-like nanoparticles show promising promise in targeted drug delivery. We provide virus-like drug conjugation services to meet your unique project needs. Our comprehensive VDC platform will provide quality services to assist our clients in drug development.
Find out more with Drug Conjugation Services.
Introduction
Targeted delivery has long been most challenging for improving the treatment of diseases. Nanoparticles (NPs) as targeted delivery vehicles for therapeutic cargo have been examined. The virus-like particles (VLPs), the emerging technology for targeted therapeutic delivery, are protein-based NPs derived from viral capsids that have the potential to overcome the limitations of other NPs, such as particle instability, slow and nonuniform drug release, and potential immunogenicity. VLPs can be classified as: animal virus-based VLPs (e.g. human papillomavirus (HPV), hepatitis B virus (HBV)), bacteriophage-based VLPs (e.g. MS2, Qβ, P22), and plant virus-based VLPs (e.g. cowpea chlorotic mottle virus (CCMV), cowpea mosaic virus (CPMV)).
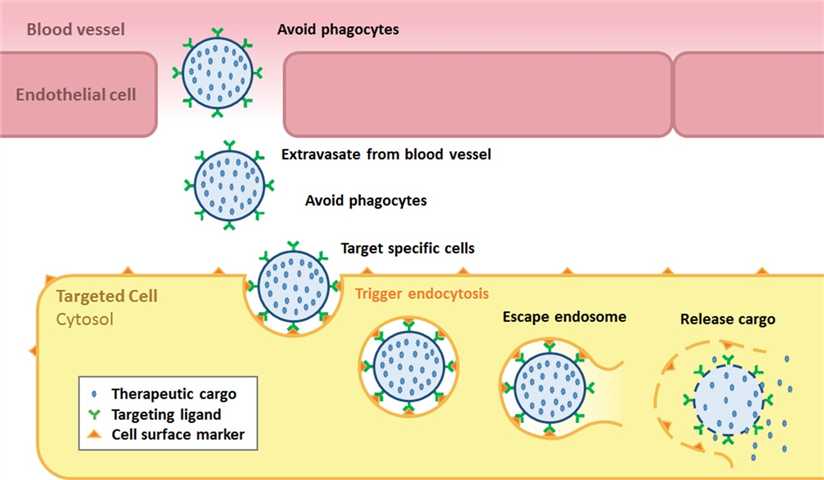 Fig. 1 Targeted delivery of VLPs (Rohovie, 2017).
Fig. 1 Targeted delivery of VLPs (Rohovie, 2017).
Virus-like drug conjugates (VDCs) use VLP as an efficient delivery vehicle to deliver payloads such as chemotherapeutic drugs, siRNA, RNA aptamers, proteins, peptides, and fluorescent probes. Based on the ability of VLPs to selectively recognize many targets, VDCs have the potential to kill cancer cells without harming normal cells.
The advantages of VDCs:
- can load small molecules, nucleic acids, and proteins
- can deliver numbers of payloads in a single VDC
- can be functionalized on the surface of VLP
- can selectively recognize overexpressed targets on cancer cells
- limiting off-target toxicity
Our Methods
- The VLP surface can be extensively modified with various biomolecules to achieve specific cellular targeting and reduce immune responses.
- The VLPs have been used to load a range of molecules by covalent and noncovalent methods. Most covalent methods for cargo loading take advantage of reactive amino acids, some use genetic fusions to the primary amino acid sequence.
| Conjugation methods | Cargo | VLPs |
| Electrostatic adsorption | RNA, DNA, Proflavin, Acridine orange | HBVc, CCMV, CPMV |
| Passive encapsidation | Green fluorescent protein, Horseradish peroxidase | HBVc, CCMV, CPMV |
| Genetic fusion | Nuclease, RNA, CRISPR, GFP | HBVc, MS2, P22, CCMV |
| Conjugated to cysteines | Taxol, Fluorescent probes, Porphyrin | MS2, P22, CPMV |
| Conjugated to tyrosines | Fluorescein | MS2 |
| Conjugated to aspartates or glutamates | Doxorubicin | CPMV |
| Conjugated to stem-loop RNA | Doxorubicin, Ricin toxin, HIV-1 Tat peptide, Quantum dot | MS2 |
| Click chemistry | Methacrylate, Gd(DOTA) | Qβ, CCMV |
| Adsorption to stem-loop RNA | Fluorescent proteins, Luciferase | Qβ |
| Coordinated by genomic RNA | Gd(III), Tb(III) | CPMV |
Applications of VDC
Currently, efforts toward to stabilize VLPs, avoid phagocytes, target specific cells, escape endosome, and release cargo still need to be continued. Although VDC targeted drug delivery remains a nascent technology that requires further studies to prove its clinical efficacy, significant progress has been made. At the recently concluded 2022 ASCO Annual Meeting, a novel virus-like drug conjugate, AU-011, was disclosed to be in clinical development in combination with an immune checkpoint inhibitor for the treatment of early-stage choroidal melanoma. This combination therapy had an effect not only on primary tumors but also on distant.
Our Advantages
- Optional VDC targeted delivery cargo
- Various VLP surface functionalized methods
- A wide range of characteristic analysis
- People and suites that specialize in VDC
- Data analysis, detailed report with results and discussion
- Cost-effective and high-quality products
Frequently Asked Questions (FAQ)
Virus-like particles (VLPs) are engineered to mimic viruses without replication. They act as carriers to deliver active compounds to specific targets, providing a controlled and efficient delivery mechanism.
VDC utilizes VLPs for precise targeting, ensuring that active compounds reach their intended locations, enhancing efficacy while reducing unwanted side effects.
VLPs are versatile and can carry peptides, proteins, small molecules, and nucleic acids, making them suitable for a broad range of applications in biotechnology.


Reference
- Rohovie, M. J., Nagasawa, M., and Swartz, J. R., Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery, Bioeng. Transl. Med., 2017, 2, 43-57.