Glycosylation
Glycosylation forms a pivotal link in biopharmaceutical research and development, harnessing the power of glycobiology to drive innovations. As a professional biotech company, BOC Sciences offers comprehensive glycosylation services. Our mission is to empower researchers, pharmaceutical professionals, and clinical laboratories with streamlined solutions of glycan conjugation, enabling them to further their understanding of various diseases, devise more effective treatments, and ultimately, enhance patient outcomes.
What are glycans?
Glycans, also known as polysaccharides, are complex carbohydrates that are present in all living organisms. These molecules are composed of sugar units, typically including monosaccharides such as glucose, mannose, and galactose. Glycans can be highly branched or linear, and their diversity is further expanded by the presence of different sugar monomers. Glycans are chemically defined as polyhydroxy aldehydes, polyhydroxy ketones and their derivatives, or polymers that produce such compounds when hydrolyzed. They have a wide range of biological functions, including cell-cell interaction, immune response, inflammation, and are essential components of many biological molecules such as protein and lipids. They can be found in a free form or attached to other molecules, forming glycoproteins or glycolipids.
What is Glycoconjugates?
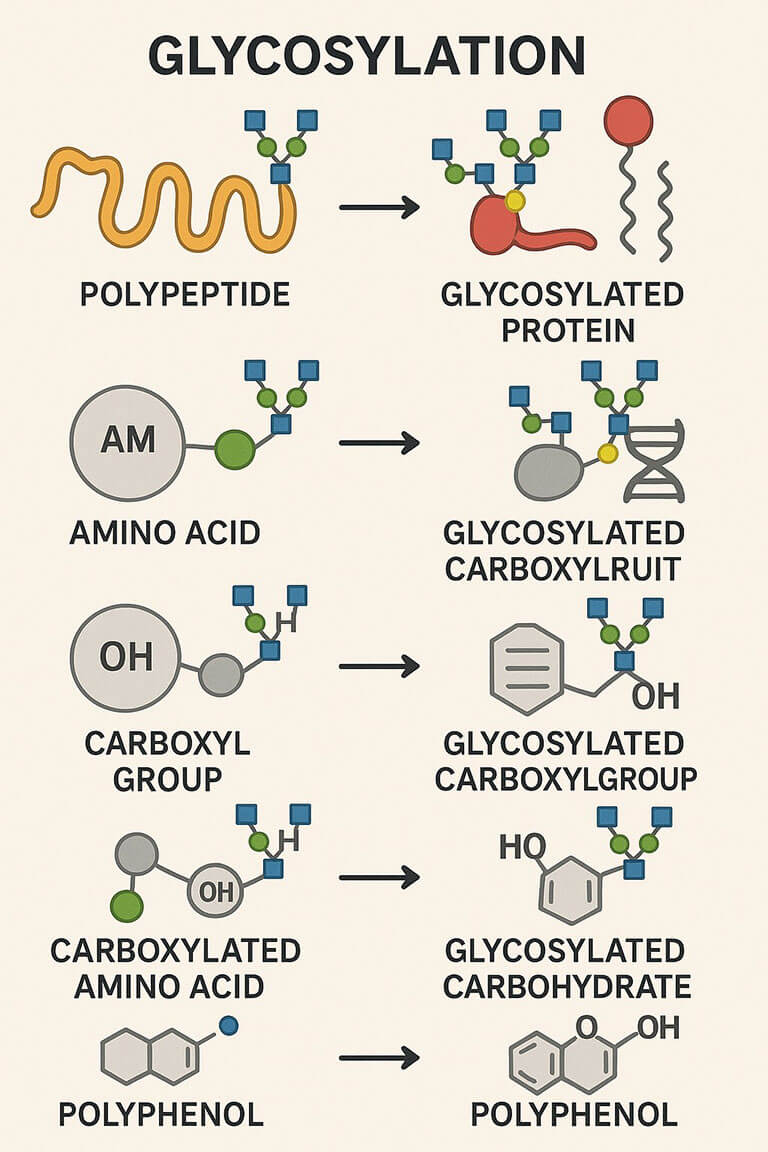
Glycosylation is a form of post-translational modification that involves adding glycan (saccharide) molecules to proteins, lipids, or other organic molecules to form glycoconjugates. This modification aids in the improved functioning of these biomolecules, increasing their stability, immunogenicity, and pharmacokinetics. Glycoconjugates are the main components of cells, formed by the covalent conjugation of glycans with proteins, lipids, or other organic molecules, and are usually expressed on the cell surface. Natural glycoconjugates play crucial roles in cellular communication, immune responses, and various physiological processes. The synthesis of glycoconjugates also has broad application prospects in biological research and therapy, including vaccines, glycan-based adjuvants, glycan conjugated antibodies, glycan-mediated drug delivery, etc.
 Fig. 1 Glycoconjugates and their biological relevance. (Pergolizzi, G., 2016)
Fig. 1 Glycoconjugates and their biological relevance. (Pergolizzi, G., 2016)
Why Need Glycan Conjugation?
Glycan conjugation can greatly increase the stability of biotherapeutics or drugs, allowing them to maintain their structure and function for a longer period of time. For example, glycans can shield therapeutic proteins from proteolytic enzymes, thereby improving their stability.
Conjugation with specific glycan structures can allow a drug to be more effectively targeted to specific cells or tissues.
Some drugs can trigger a strong immune response, leading to side effects and reducing drug efficacy. Glycan conjugation can help to reduce the immunogenicity of these substances.
Glycan conjugation can increase the solubility of bioactive compounds in water and improve their bioavailability and absorption rate. At the same time, it can prolong the biological half-life of therapeutic drugs in vivo, which means that drugs can stay in the system for a longer time, generating the advantages of administration and reducing the need for frequent administration, thus improving the compliance of patients.
Our Glycan Conjugation Services
Tailored glycan and glycoprotein engineering solutions to support therapeutic development, functional studies, and glycosylation pathway optimization.
We offer high-purity glycan synthesis using chemical and enzymatic approaches to produce oligosaccharides and polysaccharides with defined structures and sequences. These custom glycans are ideal for structural studies, glycan microarray development, vaccine design, and drug intermediate synthesis. Our services support glycoscience research with a diverse array of tailored carbohydrate molecules and tools.
Utilizing in vitro protein synthesis systems (e.g., cell-free expression platforms) and in vivo expression systems (such as mammalian cells, insect cells, and yeast), we generate recombinant glycoproteins with natural or engineered glycosylation patterns. These glycoproteins are suitable for therapeutic development, disease model creation, and bioactivity research.
We develop glycoengineered microbial strains (e.g., E. coli, Saccharomyces cerevisiae) through advanced genetic modification to optimize their glycosylation pathways. These strains enable cost-effective, large-scale production of glycosylated proteins with defined bioactivity, improving efficiency and lowering manufacturing costs in bioproduction.
Our custom glycosylation services provide tailored solutions based on specific client requirements. We design glycan structures, select modification sites, and optimize conjugation strategies to meet unique research and development goals across biopharmaceuticals, diagnostics, and functional biology applications.
End-to-end glycosylation analysis, modification, and functional assessment services for proteins and biomolecules across research and biopharmaceutical applications.
N-glycosylation refers to the attachment of glycans to the amide nitrogen of asparagine (Asn) residues in proteins. Our N-glycosylation analysis services focus on identifying glycosylation sites, characterizing glycan structures and compositions, and determining their distribution across protein sequences.
O-glycosylation involves the attachment of glycans to the hydroxyl groups of serine (Ser), threonine (Thr), or tyrosine (Tyr) residues. Common modifications include O-GalNAc and O-GlcNAc.
C-glycosylation refers to the covalent attachment of sugar moieties to the thiol group of cysteine (Cys) residues. Our C-glycosylation analysis services are designed to detect and characterize these less common modifications, evaluating their site specificity and functional implications in protein conformation, stability, and biological activity.
Protein glycosylation involves the modification of target proteins by attaching specific glycan structures either in vitro or within cellular environments. This can be achieved through chemical synthesis, enzymatic catalysis, or metabolic engineering. By introducing defined glycans, the biological functions, stability, solubility, and immunogenicity of proteins can be precisely modulated.
Peptide glycosylation refers to the synthesis of glycopeptides by adding carbohydrate moieties to peptide molecules. This modification enables in-depth studies of glycan–peptide interactions, and the roles of glycosylated peptides in cellular signaling and recognition.
Nucleic acid glycosylation involves the attachment of glycans to oligonucleotides or nucleotides, offering innovative approaches for modifying nucleic acid molecules. These glycosylation strategies are designed to influence the structural properties, stability, and biological activity of nucleic acids.
Glycolipids are biomolecules composed of a carbohydrate moiety covalently linked to a lipid. Found predominantly on cell membranes, glycolipids play essential roles in cell recognition, signal transduction, and immune response.
High-throughput, structure-specific glycan profiling and characterization using advanced MS and chromatography techniques for research and quality control.
Our high-throughput glycan analysis leverages advanced mass spectrometry techniques (such as MALDI-TOF MS and ESI-MS) and chromatography platforms (including HPLC and GC) to deliver rapid, accurate qualitative and quantitative insights into glycan structures and compositions.
Using a combination of analytical techniques such as mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and X-ray crystallography, we provide in-depth structural characterization of glycans. Our analyses cover glycosidic linkage types, branching patterns, chain lengths, and modifications, offering critical insights into glycan function and structure relationships.
By profiling glycan expression under different physiological or pathological conditions, we identify potential glycan-based biomarkers for disease diagnosis, monitoring, and treatment evaluation. Glycan analysis plays a vital role in biomarker research for cancer, autoimmune disorders, cardiovascular diseases, and more, offering new opportunities for early detection and personalized medicine.
We provide comprehensive analysis of glycan modifications, including sulfation, phosphorylation, acetylation, and methylation. Our services assess the type and site-specific distribution of glycan modifications, enabling researchers to explore their roles in key biological processes such as cell signaling, adhesion, and immune regulation.
From Inquiry to Completion: Our Service Flow
Advantages of Working with Us

- Allows the design of custom glycan conjugation strategies that meet the goals and specifications of a research or development effort
- Custom chemical conjugation and advanced enzymatic conjugation technology
- Elucidate glycoconjugate structures to ensure the accuracy of the conjugation process
- Ability to scale up the glycan conjugation process
- Integrating glycoconjugates into drug development
- Comprehensive quality control ensures the accuracy and stability of services
- Experienced expert technical team, with rich experience in the field of glycan conjugation
- The simplified polysaccharide combination service method enables us to provide results faster without affecting the quality
- Providing this highly specialized service at competitive prices
Where Our Glycosylation Are Used?
| Application of Glycoconjugates | Glycan Conjugation Service Portfolio | Service Content |
|
|
Case Study
Case Study 1
Chemically modified allergens (allergoids) are hypoallergenic preparations widely used in allergen-specific immunotherapy (AIT) due to their excellent safety profile and clinical efficacy. The suitability of coupling allergoids to mannan by means of glutaraldehyde has been demonstrated for pollen and mite allergens. By using this methodology a remarkable structural stability of allergoids conjugated to mannan is achieved, which is an important issue from the perspective of vaccine development. Next, the in vivo immunogenicity of allergoids conjugated to nonoxidized mannan were assessed in different animal models. In rabbits, polymerized allergoids conjugated to mannan (PM) induced the production of potent IgG blocking antibodies. Humoral and cellular responses have been assessed in mice following subcutaneous or sublingual administration. By either route, PM was more efficient than native allergen extracts or polymerized allergoids, to increase IgG2a/IgE and IFN-γ/IL-4 ratios, reflecting a Th1-type driven response. Importantly, an increase of FOXP3+ Treg and IL-10-producing cells was observed in mice immunized with PM as compared with polymerized allergoids. Collectively, these data demonstrate that the better uptake of allergoids conjugated to nonoxidized mannan as well as the immunomodulating features exerted on DCs are observed for different allergen conjugates, DC subsets and animal species. Allergoids conjugated to nonoxidized mannan might well represent next generation vaccines that enhance the allergen uptake by DCs and promote healthy immune responses to allergens.
 Fig.2 Steps for the generation and development of allergoids conjugated to mannan as vaccines for AIT. (Benito-Villalvilla, C., 2018)
Fig.2 Steps for the generation and development of allergoids conjugated to mannan as vaccines for AIT. (Benito-Villalvilla, C., 2018)
Case Study 2
Won et al. presented a switchable interface prepared by modifying AuNPs with polymeric "gates", which either allow the lectin to bind to the glycan present on AuNPs or not. Two different polymer chains were immobilized on the AuNPs with a size of 60 nm, a shorter one with an attached glycan and a longer one acting as an active gate. Under critical temperature, the longer polymer chain had an extended conformation with its shrinkage upon a shift of temperature above the critical value of 40 ℃, exposing underlying glycans. Lectins were detected with LOD down to µg/mL. The synthetic glycosylated nanoparticles that contain polymeric 'gates' to enable external control (via temperature changes) of glycan surface expression, as an alternative to enzymatic control in nature. This approach offers a new dynamic multivalent scaffold for glycan recognition.
 Fig.3 (A) Synthesis of polymers by RAFT. (B) Concept of using responsive polymers to gate access to nanoparticles. (C) UV-Vis traces of different nanoparticle formulations in presence of serial dilution of lectin (1-10 µg/mL) after 30 min incubation at 20 ℃ or 40 ℃. (D) An increase in Abs700 and decrease in Abs540 is indicative of binding. TEM images of these particles after addition of lectin (E) at 20 ℃ for 30 min; (F) at 40 ℃ following 30 min incubation. (Won, S., 2017)
Fig.3 (A) Synthesis of polymers by RAFT. (B) Concept of using responsive polymers to gate access to nanoparticles. (C) UV-Vis traces of different nanoparticle formulations in presence of serial dilution of lectin (1-10 µg/mL) after 30 min incubation at 20 ℃ or 40 ℃. (D) An increase in Abs700 and decrease in Abs540 is indicative of binding. TEM images of these particles after addition of lectin (E) at 20 ℃ for 30 min; (F) at 40 ℃ following 30 min incubation. (Won, S., 2017)
Frequently Asked Questions (FAQ)
Many different types of drugs, especially proteins-based drugs, like antibodies and vaccines, can benefit from glycan conjugation. This is because glycan conjugation can modulate the behavior of these proteins in the body to improve their therapeutic effectiveness.
We leverage both traditional and novel bioconjugation techniques including enzymatic and chemical conjugation methods. The choice of method depends on the structure and properties of the substance to be conjugated.
We follow stringent quality control measures to ensure the highest standard of our glycan conjugation services. These include rigorous checks throughout the conjugation process, detailed documentation for reproducibility, and state-of-the-art analytical services to verify the quality of the conjugates.
Although the conjugation is typically designed to be stable and non-reversible under physiological conditions, there are certain enzymatic and chemical methods by which the conjugation can be reversed. However, the feasibility and success of de-conjugation largely depend on the specifics of the conjugate and method of attachment.
Absolutely. We understand that each project has unique requirements. Therefore, we offer personalized solutions and work closely with our clients to optimize the glycan conjugation process according to their specific needs.


Learn More About Glycosylation
References
- Pergolizzi, G., et al. Contemporary glycoconjugation chemistry. Carbohydrate Chemistry. 2016, 42: 1-46.
- Lang, S.; Huang, X. Carbohydrate conjugates in vaccine developments. Frontiers in Chemistry. 2020, 8: 284.
- Benito-Villalvilla, C., et al. Novel vaccines targeting dendritic cells by coupling allergoids to mannan. Allergo Journal International. 2018, 27: 256-262.
- Won, S., et al. Externally controllable glycan presentation on nanoparticle surfaces to modulate lectin recognition. Nanoscale Horizons. 2017, 2(2): 106-109.