Oligonucleotide-Loaded Lipid Nanoparticle
As a leading CRO in the fields of drug development and drug delivery, BOC Sciences offers LNP encapsulation services for oligonucleotides to meet the growing demands in oligonucleotide drug development. Based on our expertise in oligonucleotide conjugation, we are able to provide complete solutions to facilitate your project.
Lipid Nanoparticle Loaded with Oligonucleotide
Oligonucleotide-loaded lipid nanoparticles (LNPs) act as barriers, helping oligonucleotide drugs evade immune system capture and nuclease degradation, reaching the target tissue. Oligonucleotides are short-chain nucleotide compounds composed of bases. Oligonucleotides represent a new modality in drug development, emerging after small molecule drugs and protein drugs. Due to the high hydrophilicity and negative charge of oligonucleotide molecules, they do not easily traverse cell membranes and the blood-brain barrier, and are susceptible to nuclease degradation. LNP drug delivery technology has become a key approach in oligonucleotide drug development, enabling the delivery of active ingredients to targets within the complex in vivo environment. The positive charge carried by lipid molecules can interact with the negatively charged oligonucleotide molecules, forming nanoscale lipid particles that encapsulate the oligonucleotides. With the assistance of the different functional lipid components, LNPs enable successful transmembrane crossing and endosomal escape of oligonucleotide molecules, ultimately achieving therapeutic goals through gene regulation.
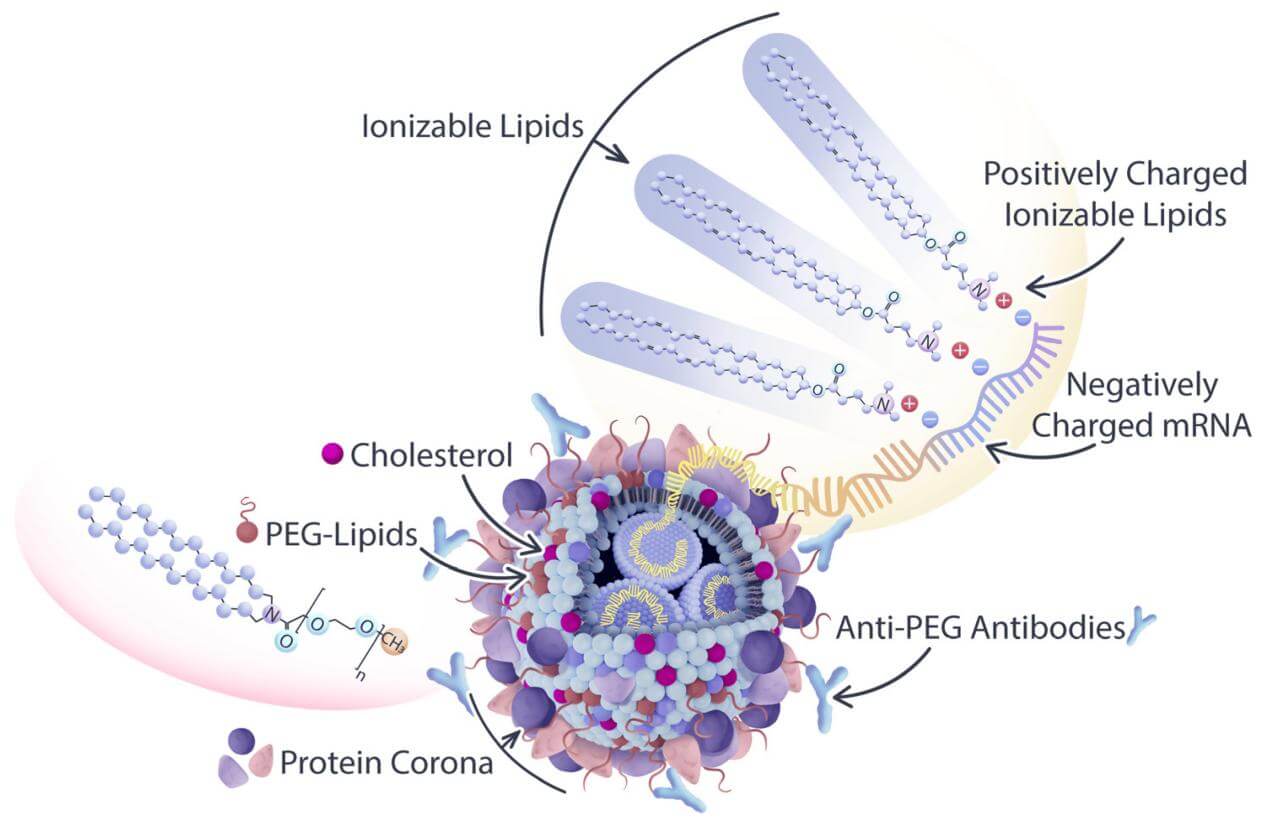 Figure 1. LNP used in oligonucleotide delivery. (Korzun, 2023)
Figure 1. LNP used in oligonucleotide delivery. (Korzun, 2023)
Oligonucleotide LNP Delivery Services
LNPs have emerged as a leading technology for achieving efficient and targeted delivery of therapeutic oligonucleotides. Our services for LNP loaded with oligonucleotides offer a comprehensive solution, including modifying oligonucleotides and encapsulating, formulating, and optimizing LNPs for gene therapy, RNA-based therapeutics, and molecular medicine.
- Oligonucleotide modification
- Oligonucleotide lipid conjugation
- Custom lipid formulation design
- Incorporation of PEGylation
- Surface ligand functionalization
- Stability enhancement
- Active loading techniques
- Passive encapsulation methods
- Physicochemical characterization
- Stability studies
- Cellular uptake studies
- Pharmacokinetics studies
- Formulation characterization documentation
- Scale-up and manufacturing solutions
What are Lipid Nanoparticles?
Lipid nanoparticles are nano-sized particles composed primarily of lipids, which are essentially fatty molecules. LNPs can encapsulate drugs within their core or even within the lipid layer itself. Various lipid components of LNPs work together to encapsulate and protect therapeutic payloads.
- Phospholipids: These are the primary structural elements of the lipid bilayer in LNPs. Phospholipids, such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE), form the outer and inner layers of the lipid membrane.
- Cholesterol: Cholesterol is often included in LNP formulations to enhance membrane rigidity and stability.
- Cationic Lipids: Cationic lipids are crucial for facilitating interactions with negatively charged oligonucleotides.
- PEGylated Lipids: PEGylation reduces recognition by the immune system and can contribute to prolonged circulation.
- Ionizable Lipids: Ionizable lipids are designed to carry a positive charge at low pH, such as the mildly acidic environment found within endosomes, facilitating endosomal escape.
- Lipid Anchors: Some LNPs may include lipid anchors, which can be covalently attached to the therapeutic oligonucleotides.
- Neutral Lipids: Neutral lipids, such as monoacylglycerols and diacylglycerols, can be added to adjust the overall lipid composition and stability of LNPs.
- Fluorescent or Radioactive Lipids: These lipid molecules can be incorporated as tracers to monitor the distribution of LNPs.
Lipid Nanoparticle Encapsulation
The encapsulation of oligonucleotides within LNPs requires precision and innovation. Various techniques are employed to achieve efficient encapsulation, including emulsification, solvent evaporation, and self-assembly. Emulsification involves mixing the drug and lipid components in a solvent to form a stable emulsion, which is then processed to create nanoparticles. Solvent evaporation, on the other hand, relies on gradually removing the solvent from a preformed emulsion, resulting in the formation of nanoparticles. Self-assembly utilizes the inherent properties of lipids to form nanoparticles spontaneously under specific conditions. These techniques ensure the creation of lipid nanoparticles with optimal drug-loading capacities and desired release profiles.
Lipid-Oligonucleotide Conjugates
Lipids and oligonucleotides can be united through chemical conjugation, resulting in lipid-oligonucleotide conjugates. The lipid portion can include cholesterol, phospholipids, fatty acids, or lipophilic molecules. The attachment of the lipid moiety to the oligonucleotide can occur through various chemical linkages, including phosphodiester bonds, phosphorothioate bonds, or click chemistry approaches.
Lipid Nanoparticle Delivery
LNPs can assist oligonucleotide molecules in crossing biological barriers and releasing in specific physiological environments, thereby enhancing the delivery and potency of oligonucleotide molecules. The majority of oligonucleotides recognize and bind to target nucleotide sequences through Watson-Crick base pairing principles, disrupting the transcription and translation of disease-causing genes, thereby correcting the expression levels of pathogenic proteins and ultimately achieving therapeutic effects. Based on differences in structure and mechanisms of action, oligonucleotide drugs can be broadly categorized into antisense oligonucleotides (ASOs), small interfering RNA (siRNA), microRNA (miRNA), and nucleic acid aptamers, among others. Currently, two mRNA COVID-19 vaccines utilized LNPs to encapsulate and deliver mRNA instructions that encode the spike protein of the SARS-CoV-2 virus. In the realm of siRNA therapeutics, LNPs have exhibited remarkable potential. The encapsulation of siRNA within lipid nanoparticles not only protects the fragile siRNA molecules but also facilitates their efficient uptake by target cells.
Frequently Asked Questions (FAQ)
Oligonucleotide-loaded lipid nanoparticles (LNPs) offer enhanced stability, targeted drug delivery, and reduced side effects. They protect oligonucleotides from degradation and improve cellular uptake.
LNPs facilitate the crossing of cell membranes through their lipid composition, promoting endocytosis and enhancing drug efficacy, especially in gene therapies and RNA-based treatments.
The lipid composition of LNPs determines encapsulation efficiency, release profile, and stability. By modifying the lipid materials, such as using cationic or PEGylated lipids, the delivery of specific oligonucleotides can be optimized.


Reference
- Korzun, T., et al., From Bench to Bedside: Implications of Lipid Nanoparticle Carrier Reactogenicity for Advancing Nucleic Acid Therapeutics, Pharmaceuticals, 2023, 16(8), 1088.