Fluorescent Liposome Labeling Services
Enterprise-GradeFor High-Confidence TrackingReproducible Lipid Labeling & Liposome Formulation Support
Accelerate R&D and translation with enterprise-ready fluorescent liposome solutions designed for biotechnology, pharmaceutical development, advanced drug delivery, diagnostics, and CRO programs. Fluorescent liposomes enable quantitative, visualizable tracking of lipid nanocarriers in complex workflows—supporting uptake studies, biodistribution research, release kinetics evaluation, formulation optimization, and assay development. Using established lipid conjugation and incorporation approaches (e.g., fluorescent lipid analog incorporation, bilayer insertion strategies, and controlled encapsulation of fluorescent tracers where appropriate), we help teams generate labeled liposomes with strong signal clarity while maintaining critical formulation attributes such as size distribution, encapsulation performance, and colloidal stability.
Choose from single-color labeling for routine tracking, multi-color designs for comparative studies (e.g., co-localization or multi-formulation screening), or customized fluorescent liposome architectures aligned to your analytical platform (fluorescence microscopy, flow cytometry-based uptake, plate-reader assays, fluorescence spectroscopy, and compatible in vivo imaging workflows when suitable fluorophores are selected). Each project is designed around measurable, application-relevant criteria—particle size/PDI targets, fluorescence readout requirements, stability expectations, and downstream compatibility—supporting robust decision-making across development stages.
What Are Fluorescent Liposomes?
Fluorescent liposomes are lipid bilayer vesicles engineered with fluorescent reporters to enable tracking, visualization, and quantification of liposomal nanocarriers in biological and formulation studies. Labeling is commonly achieved by incorporating fluorescent lipid analogs into the membrane (for membrane tracking), inserting lipophilic dyes into the bilayer (for post-formulation labeling in appropriate cases), or encapsulating fluorescent tracers in the aqueous core (for release and leakage assessments). These approaches support enterprise use cases such as formulation screening, cellular uptake profiling, stability studies, interaction testing with serum proteins, and method development for imaging or plate-based assays—while emphasizing control of core quality attributes (e.g., size, PDI, zeta potential, and signal stability) so fluorescent readouts remain interpretable and reproducible.
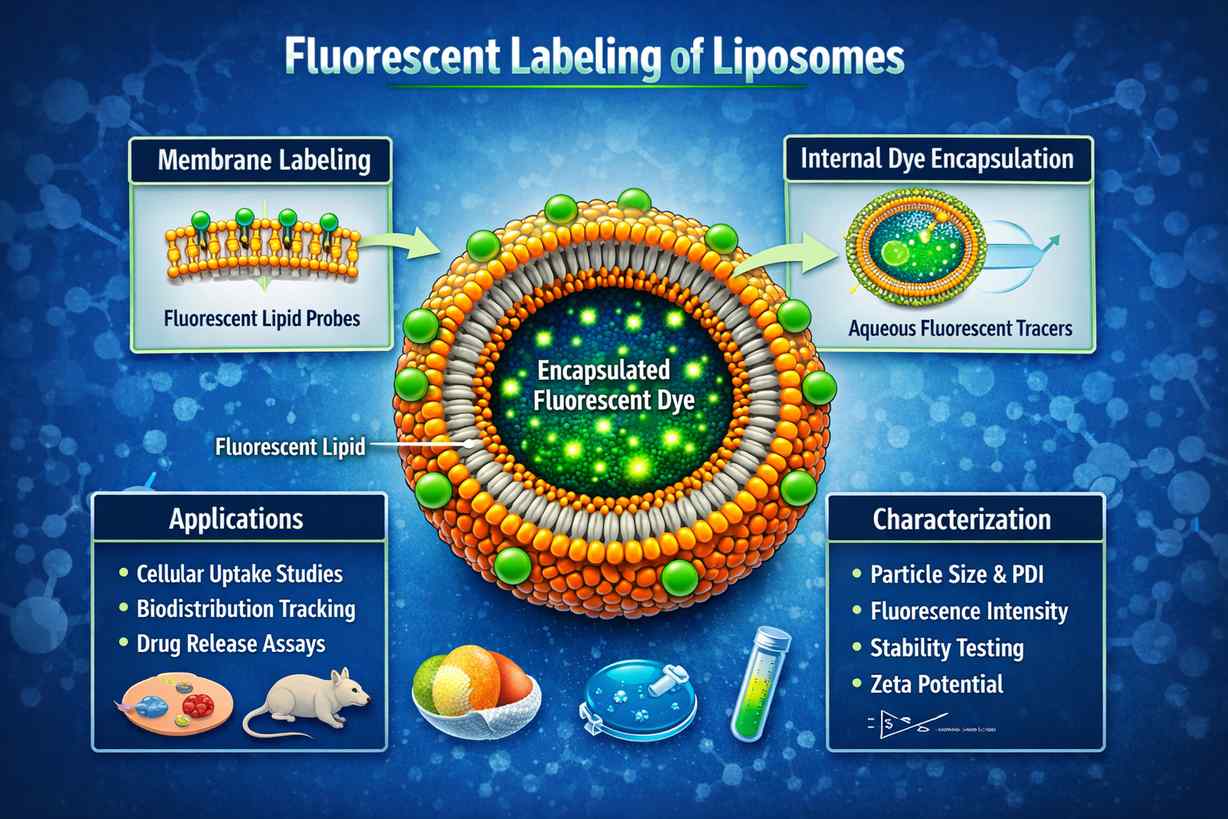 Fluorescent liposomes labeled via membrane-integrated lipid dyes and encapsulated tracers, enabling visualization, uptake studies, biodistribution tracking, and release characterization in drug delivery and nanomedicine research.
Fluorescent liposomes labeled via membrane-integrated lipid dyes and encapsulated tracers, enabling visualization, uptake studies, biodistribution tracking, and release characterization in drug delivery and nanomedicine research.What Problems We Solve
We implement membrane-associated labeling strategies that enable reliable visualization and quantification of liposome behavior in uptake, trafficking, and comparative formulation studies.
We reduce non-specific fluorescence by emphasizing free-dye cleanup and workflow design, helping ensure measured signal reflects liposome-associated fluorescence rather than residual dye.
We select labeling modes and dye formats aligned to study goals (membrane fate vs. release) and verify performance with fluorescence characterization and stability checks under defined conditions.
We prioritize formulation-aware labeling to preserve critical quality attributes such as particle size distribution and surface behavior, supporting meaningful comparison to unlabeled controls.
We design multicolor fluorescent liposome sets with practical channel planning to minimize signal cross-talk, supporting co-localization and comparative tracking workflows.
For leakage and release studies, we support tracer-encapsulated designs that enable quantitative fluorescence-based integrity testing and comparative release profiling.
Our Fluorescent Liposome Services
We provide a focused portfolio of fluorescent liposome services designed for enterprise research, formulation development, and nanomedicine programs. Each service is built around established liposome preparation and labeling practices, with an emphasis on reproducibility, formulation integrity, and application-relevant fluorescence performance. Our offerings support industrial R&D teams across feasibility studies, comparative screening, assay development, and mechanistic investigations.
 Membrane-Labeled Fluorescent Liposomes
Membrane-Labeled Fluorescent Liposomes
Capabilities include:
- Incorporation of fluorescent lipid analogs (e.g., NBD- or rhodamine-labeled phospholipids) during liposome formulation
- Bilayer labeling using lipophilic dyes for post-formation membrane tracking
- Support for neutral, cationic, anionic, and PEGylated liposome systems
- Size-controlled liposomes prepared via extrusion or equivalent methods
- Multicolor membrane labeling for comparative formulation or uptake studies
- QC covering size, PDI, surface charge, and fluorescence performance
- Compatibility with microscopy, plate-reader assays, and uptake studies
Typical dyes:
DiI, DiD, DiR, NBD-lipids, rhodamine-labeled lipids, BODIPY dyes
 Encapsulated Fluorescent Liposomes (Tracer-Based)
Encapsulated Fluorescent Liposomes (Tracer-Based)
Capabilities include:
- Encapsulation of water-soluble fluorescent tracers for leakage and release studies
- Self-quenching tracer systems for sensitive membrane integrity assessment
- Cleanup and removal of external/free dye to reduce background signal
- Support for stress, serum, or time-course release evaluations
- Comparative encapsulation efficiency and retention assessments
- Size and fluorescence stability tracking over defined storage conditions
- Data packages aligned to formulation screening and optimization workflows
Typical tracers:
Calcein, fluorescein derivatives, rhodamine-based aqueous dyes
 Multicolor & Comparative Fluorescent Liposome Panels
Multicolor & Comparative Fluorescent Liposome Panels
Capabilities include:
- Design of spectrally distinct fluorescent liposome sets for side-by-side comparison
- Parallel labeling of multiple formulations or surface chemistries
- Optimization to minimize spectral overlap and signal cross-talk
- Support for co-localization, competitive uptake, and formulation ranking studies
- Documentation of dye selection and fluorescence settings for reproducibility
- QC consistency checks across all panel members
Typical dyes:
DiO, DiI, DiD, DiR, NBD-lipids, rhodamine-lipids
 Custom Fluorescent Liposome Development
Custom Fluorescent Liposome Development
Capabilities include:
- Custom labeling strategy selection based on study objectives and analytical platforms
- Integration of fluorescent liposomes into design-of-experiments frameworks
- Iterative optimization of labeling density and formulation parameters
- Method development support for fluorescence-based liposome assays
- Adaptation to internal SOPs or cross-team reproducibility requirements
- Transparent QC documentation to support internal review and collaboration
Project focus:
Enterprise R&D, formulation screening, nanomedicine development, assay validation
Fluorophores We Offer
For enterprise fluorescent liposome programs, fluorophore selection is driven by measurable performance in lipid bilayers and relevant biological matrices. We support both membrane-labeling (lipid analog incorporation or bilayer insertion) and aqueous-core tracers (for leakage/release studies) using widely adopted dyes with well-characterized spectra and established use in liposome and membrane research. Selection is guided by your detection platform (microscopy, plate readers, flow-based uptake assays, spectroscopy, and compatible in vivo imaging workflows) and by formulation risk factors such as dye self-quenching, redistribution, photostability, and impact on size/PDI.
| Fluorophore / Lipid Dye | Excitation (nm) | Emission (nm) | Typical Applications | Formulation Considerations |
| DiO (3,3'-Dioctadecyloxacarbocyanine) | 484 | 501 | Membrane tracking, liposome uptake studies, comparative formulation screening | Lipophilic bilayer dye; useful for robust membrane signal; post-insertion or membrane incorporation depending on workflow |
| DiI (DiIC18(3)) | 549 | 565 | Liposome membrane labeling for fluorescence microscopy and uptake visualization | Strong membrane-associated signal; commonly used carbocyanine dye for lipid bilayers |
| DiD (DiIC18(5)) | 644 | 665 | Far-red membrane labeling, multicolor liposome tracking, reduced background imaging | Far-red channel can reduce autofluorescence; suitable for multiplex designs with careful spectral planning |
| DiR | 750 | 782 | Near-IR liposome tracking for deep-tissue imaging workflows and biodistribution studies | NIR signal supports low-background imaging; selection should consider matrix effects and instrument filters |
| NBD-PE (NBD-labeled phosphatidylethanolamine) | 463 | 536 | Lipid dynamics studies, membrane organization research, GUV/liposome labeling | Fluorescent lipid analog incorporated during formulation; supports mechanistic lipid behavior studies |
| Rhodamine DHPE (Rhodamine B-labeled phosphatidylethanolamine) | 560 | 580 | Red-channel membrane labeling for microscopy, co-localization, formulation comparison | Headgroup-labeled lipid probe; typically incorporated during lipid mixing/film preparation |
| BODIPY FL | 503 | 512 | Bright green-channel tracking for liposome assays and microscopy | High brightness with fluorescein-like spectra; selection should consider bilayer partitioning and assay format |
| Calcein (aqueous tracer) | 495 | 515 | Encapsulation-based leakage/release assays, membrane integrity testing | Self-quenching at high concentrations enables sensitive leakage readouts; used for release/retention characterization |
Our Labeling Chemistry & Methods
Fluorescent liposome labeling requires strategies that preserve critical quality attributes while producing interpretable fluorescent readouts. In practice, enterprise programs typically select from membrane-incorporated fluorescent lipid analogs, post-insertion/bilayer-partitioning dyes, or encapsulated aqueous tracers (for leakage and release). Strategy selection is based on what you need to measure (membrane fate vs. cargo leakage vs. comparative formulation behavior), your downstream assay conditions (serum exposure, washing steps, imaging duration), and your acceptance criteria for size/PDI stability.
| Labeling Strategy | Chemistry / Mechanism | Common Applications | Advantages |
| Fluorescent Lipid Analog Incorporation | Fluorescently labeled lipids (e.g., NBD-PE, rhodamine-labeled PE) are blended with bulk lipids prior to liposome formation, embedding the reporter in the bilayer. | Membrane tracking, lipid mixing studies, formulation comparison, bilayer dynamics research | Controlled incorporation with predictable bilayer localization; suitable for reproducible, batch-to-batch comparable membrane signal |
| Bilayer Insertion (Lipophilic Dye Partitioning) | Lipophilic dyes (e.g., carbocyanines such as DiI/DiD/DiR) partition into lipid bilayers and provide strong membrane-associated fluorescence. | Rapid membrane labeling, uptake and trafficking studies, imaging-based screening | Fast implementation for existing liposomes; strong signal; compatible with multicolor designs when spectra are planned appropriately |
| Aqueous-Core Encapsulation (Tracer Loading) | Water-soluble fluorophores (e.g., calcein) are encapsulated in the aqueous core during hydration/formation; leakage is measured via fluorescence changes after release. | Leakage/release assays, membrane integrity testing, stability studies under stress or serum exposure | Directly supports release/retention measurements; widely used for integrity and leakage characterization |
| Fluorescent Surface Component Integration | Fluorescent moieties can be introduced via functionalized lipids (e.g., labeled PEG-lipids or lipid-linked reporters) incorporated into the membrane to report surface behavior. | Surface presentation studies, interaction assays, comparative targeting/stealth evaluations | Enables surface-focused tracking without relying solely on bilayer-partitioning dyes; supports structured design-of-experiments for formulation optimization |
Quality Control & Data Delivered
Enterprise fluorescent liposome work requires QC that connects fluorescence performance to liposome critical quality attributes. We provide a data package designed to support R&D decision-making, reproducibility expectations, and method development needs—covering particle attributes, fluorescence characterization, and (when applicable) encapsulation/leakage performance. QC scope is selected based on your intended use (imaging, uptake, biodistribution, release testing, assay development) and your target acceptance criteria.
| QC Attribute | Analytical Method | Delivered Output |
| Particle Size & PDI | DLS (Dynamic Light Scattering) | Size distribution report, mean size, PDI |
| Surface Charge | Zeta potential analysis | Zeta potential values and measurement report |
| Fluorescence Characterization | Excitation/emission scan (spectrofluorometry) / plate-reader characterization (as applicable) | Spectral profiles, fluorescence intensity readouts under defined settings |
| Dye Incorporation / Content (membrane-labeled) | UV-Vis absorbance and/or fluorescence calibration (method selected by dye and matrix) | Estimated dye content under defined calibration approach; batch comparison data when requested |
| Free Dye / Purification Verification | Size-exclusion cleanup verification and/or fraction analysis (workflow-dependent) | Evidence of free dye reduction (fraction readouts / chromatogram-style outputs when applicable) |
| Encapsulation Performance (when aqueous tracer is used) | Post-cleanup fluorescence quantification; optional leakage challenge readouts per study design | Encapsulation/retention readouts and leakage trend data under specified conditions |
| Stability Assessment | Storage stability tracking (size/PDI and fluorescence over time) under defined conditions | Stability time-course (size/PDI + fluorescence) and handling guidance aligned to results |
| Morphology (optional) | TEM / cryo-TEM (when required by program) | Micrographs and morphology notes (optional) |
General Workflow for Fluorescent Liposome Services
We assess your liposome composition, preparation method, target size/PDI, and intended application (e.g., uptake, biodistribution, release). Based on these parameters, an appropriate fluorescent labeling strategy and dye selection are defined to ensure interpretable, application-relevant readouts.
Fluorescent lipids, membrane-partitioning dyes, or aqueous tracers are incorporated using controlled, workflow-appropriate procedures. Implementation is aligned with your formulation stage (pre- or post-formation) to minimize impact on liposome integrity.
Free or unincorporated dye is reduced using size-exclusion–based cleanup or equivalent approaches selected for the liposome format. This step ensures that observed fluorescence reflects liposome-associated signal rather than background dye.
Particle size, PDI, surface charge, and fluorescence performance are characterized to confirm that labeling has not compromised formulation attributes and that fluorescence output meets study requirements.
You receive the fluorescent liposome material with a clear QC summary and usage guidance aligned to your downstream assays, enabling confident deployment in R&D workflows.
Advantages of Our Fluorescent Liposome Services
Labeling strategies are selected with explicit consideration of lipid composition, size targets, and stability requirements, ensuring fluorescence data reflects true liposome behavior rather than artifacts.

Controlled dye incorporation and cleanup workflows support consistent fluorescence performance across batches, enabling comparative formulation and screening studies.
Fluorescent liposomes are designed for compatibility with microscopy, plate-reader assays, flow-based uptake studies, and spectroscopy-based characterization commonly used in industrial R&D.
Multicolor and tracer-based designs enable side-by-side comparison of formulations, surface chemistries, or process variables within a single experimental framework.
Applications of Fluorescent Liposome Technology
Cellular Uptake & Trafficking
- Visualize liposome internalization and intracellular distribution using fluorescence microscopy.
- Compare uptake efficiency across formulations or surface modifications.
- Assess trafficking pathways and subcellular localization.
Biodistribution & Tracking
- Track liposome fate across biological matrices using appropriate fluorescence channels.
- Support comparative biodistribution studies in preclinical research settings.
- Enable longitudinal tracking with stable membrane-associated dyes.
Formulation Optimization
- Compare lipid compositions, PEG densities, or surface chemistries using fluorescence-based assays.
- Screen formulation variables for stability, uptake, or interaction profiles.
- Support design-of-experiments approaches in liposome development.
Release & Leakage Studies
- Monitor encapsulated tracer release under stress or biological conditions.
- Evaluate membrane integrity and formulation robustness.
- Generate comparative release profiles across formulations.
Nanomedicine & Drug Delivery R&D
- Support early-stage nanocarrier evaluation with fluorescent tracking tools.
- Visualize delivery behavior in cell-based and model systems.
- Enable mechanism-focused delivery studies.
Method & Assay Development
- Develop and validate fluorescence-based assays for liposome characterization.
- Establish standardized readouts for comparative and longitudinal studies.
- Support transferability of methods across teams or programs.
What Our Research Clients Say

"The fluorescent liposomes we received were exceptionally consistent in size and signal. The membrane labeling allowed us to clearly compare uptake behavior across multiple formulations without introducing artifacts."
— Director of Drug Delivery, Global Pharmaceutical Company
"Their formulation-aware approach made a real difference. The fluorescence data correlated well with our internal stability and release studies, which streamlined our screening workflow."
— Senior Scientist, Nanomedicine Biotechnology Firm
"We needed reproducible fluorescent liposomes for assay development across teams. The QC documentation and consistency across batches met our internal standards and audit expectations."
— Assay Development Lead, International CRO
Custom Fluorescent Liposome Solutions for Enterprise R&D
Whether you are optimizing lipid formulations, developing nanocarrier-based therapeutics, or establishing fluorescence-driven assays, we provide enterprise-focused fluorescent liposome solutions aligned with real development needs. Our team works closely with pharmaceutical, biotechnology, and CRO partners to select appropriate labeling strategies, define meaningful QC criteria, and deliver fluorescent liposomes that integrate seamlessly into existing workflows.
From early-stage feasibility studies to advanced formulation screening and method development, we support data-driven decision-making with reproducible materials and transparent documentation. Contact us to discuss your project requirements or request a technical consultation, and explore how fluorescent liposomes can strengthen your research and development programs.
Frequently Asked Questions (FAQ)
Fluorescent liposomes are used to visualize, track, and quantify liposomal nanocarriers in research and development workflows. Common applications include cellular uptake studies, biodistribution tracking, formulation comparison, membrane integrity testing, and release or leakage assays. By incorporating fluorescent dyes into the lipid bilayer or encapsulating tracers in the aqueous core, researchers can monitor liposome behavior across in vitro and preclinical models using fluorescence-based techniques.
Liposomes are typically fluorescently labeled using three established approaches:
incorporation of fluorescent lipid analogs during formulation,
insertion of lipophilic dyes into the lipid bilayer after formation, or
encapsulation of water-soluble fluorescent tracers inside the aqueous core.
The choice depends on whether the study focuses on membrane tracking, surface behavior, or cargo release.
When properly designed, fluorescent labeling should not significantly alter liposome size, polydispersity, or stability. However, dye type, labeling density, and insertion method can influence membrane properties. For this reason, particle size, PDI, surface charge, and fluorescence performance are routinely evaluated after labeling to confirm formulation integrity.
Membrane-labeled liposomes carry fluorescent dyes within the lipid bilayer and are primarily used to track liposome location, uptake, and distribution. Encapsulated fluorescent liposomes contain water-soluble tracers in the aqueous core and are commonly used to study membrane integrity, leakage, and release behavior. Each approach answers different experimental questions.
Common fluorophores include carbocyanine dyes (such as DiI, DiD, and DiR) for membrane labeling, fluorescent lipid analogs (such as NBD- or rhodamine-labeled phospholipids), and aqueous tracers like calcein for release studies. Fluorophore selection depends on detection platform, spectral requirements, and compatibility with the liposome formulation.

