Bioorthogonal Reactions
Bioorthogonal reactions, which allow for the efficient and selective labeling of biomolecules in living cells and organisms, are chemical processes that can take place in complex biological systems without interfering with the natural biochemical processes and without significantly toxicating or perturbing the biological system. In the domains of biomedical imaging, pharmaceutical chemistry, protein synthesis, polymer science, and materials research, these bioorthogonal processes have shown unmatched benefits.
Bioorthogonal reactions typically fulfill several key criteria:
- Selectivity
- Fast kinetics
- Biocompatibility
- Stability
- Water solubility
The Progress of Bioorthogonal Reactions
The early 2000s saw the emergence of the concept of bioorthogonal chemistry, which aims to create chemical processes that are independent on biological elements. The term bioorthogonal refers to a response that does not adversely affect or cross-react with endogenous biomolecules, such as proteins, nucleic acids, or metabolites. Bioorthogonal reaction can occur in a biological environment with high yield, high selectivity, high efficiency, and no side reactions. Over the years, various types of bioorthogonal reactions have been made, including Staudinger ligation, copper-catalyzed alkyne-azide cycloaddition, Strain-promoted [3+2] reactions, and so on. They are widely applied in genetic code expansion techniques, metabolic engineering, drug target identificaton, antibody-drug conjugates, and drug delivery systems. Finally, In 2022, the Nobel Prize in Chemistry was awarded to three scientists who have made groundbreaking contributions to bioorthogonal chemistry.
Click Chemistry Reactions
Click chemistry reactions, such as the copper-catalyzed azide-alkyne cycloaddition (CuAAC) , were among the earliest bioorthogonal reactions to be widely utilized. These reactions involve the coupling of azide and alkyne functional groups to form stable triazole linkages. Click chemistry reactions are highly selective, efficient, and compatible with biological systems. However, the copper catalyst used in CuAAC can be toxic to cells, leading to the development of copper-free click chemistry reactions.
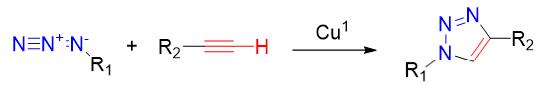
Advantages:
- High selectivity
- Mild conditions
- Efficient conjugation
- Compatibility
Disadvantages:
- Toxicity of catalyst
Strain-Promoted Reactions
Strain-promoted bioorthogonal reactions, such as the strain-promoted azide-alkyne cycloaddition (SPAAC) , have gained significant attention due to their compatibility with living systems. These reactions do not require catalysts and can proceed rapidly under physiological conditions. SPAAC utilizes strained cyclooctyne derivatives and azides to form stable triazole products. The absence of catalysts in strain-promoted reactions reduces toxicity and background signal interference.
In addition, strain-promoted alkyne-nitrone cycloaddition (SPANC) is a powerful synthetic method in organic chemistry to form cycloadducts through the reaction between an alkyne and a nitrone. This reaction is highly efficient and occurs under mild reaction conditions, which has been employed in the synthesis of natural products, pharmaceuticals, and materials with unique properties.
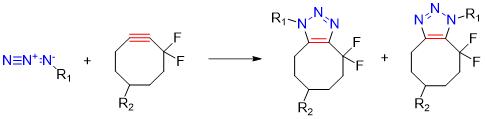
Advantages:
- Catalyst-free reaction
- Rapid kinetics
- High selectivity
- Bioorthogonal compatibility
Disadvantages:
- Strain-promoted requirement
- Structural constraints
- Click-to-release challenges
Bioorthogonal Tetrazine Ligation
Bioorthogonal tetrazine ligation is a fast and selective bioorthogonal reaction. Tetrazine reacts with a strained alkene, typically a trans-cyclooctene (TCO), forming a highly stable dihydropyrazine product. In the reaction, the strained alkene acts as the dienophile, while the tetrazine serves as the diene. This inverse electron-demand Diels-Alder (iEDDA) reaction is known for its fast reaction kinetics at room temperature, even at low concentrations, and has been widely utilized for bioconjugation, bioimaging, and protein labeling applications.
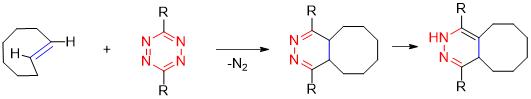
Advantages:
- Fast reaction kinetics
- Selectivity
- Stability
Disadvantages:
- Limited functional group compatibility
Staudinger Ligation
The Staudinger ligation is a bioorthogonal reaction based on the reaction between azides and phosphines. This reaction forms stable triarylphosphine oxide and amine products. Staudinger ligation is compatible with living systems and has been used for site-specific protein labeling and imaging.
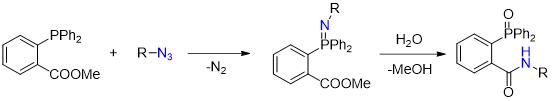
Advantages:
- Selectivity
- Versatility
- Compatibility
Disadvantages:
- Sensitivity to reaction conditions
Bioorthogonal Redox Reactions
Bioorthogonal redox reactions involve the use of bioorthogonal handles that undergo reduction or oxidation reactions. For example, the bioorthogonal handle based on the reaction between arylboronic acids and hydrogen peroxide can be used for selective protein labeling and imaging. Redox reactions provide an additional dimension to bioorthogonal chemistry and expand its applications.
Advantages:
- Regioselectivity
- Compatibility
Disadvantages:
- Specificity challenges
- Redox sensitivity
Conclusion
Bioorthogonal reactions have evolved significantly since their inception, offering researchers a diverse toolbox for studying and manipulating biological systems. These reactions enable selective labeling, tracking, and modification of biomolecules in living systems, contributing to advancements in bioimaging, proteomics, drug delivery, and glycobiology. As the field continues to advance, new bioorthogonal reactions and strategies will likely emerge, further expanding our understanding of complex biological processes.
Frequently Asked Questions (FAQ)
Bioorthogonal reactions are chemical reactions that can occur in biological systems without interfering with natural biochemical processes. These reactions are highly selective, efficient, and biocompatible, making them ideal for labeling and modifying biomolecules in living cells and organisms.
In materials science, bioorthogonal reactions are used for functionalizing surfaces, modifying polymers, and creating advanced materials with tailored properties. These reactions, such as strain-promoted azide-alkyne cycloaddition (SPAAC) and tetrazine ligation, enable precise control over material properties without disrupting the biological environment.
Bioorthogonal chemistry is preferred because it does not interfere with native cellular processes. Traditional chemical reactions can lead to unwanted side effects or toxicity, while bioorthogonal reactions maintain cellular integrity and provide high selectivity for the desired modifications without affecting biological systems.

