Tetrazine Ligation
Tetrazine ligation is a class of bioorthogonal reactions, which is the reaction of dienophile and S-tetrazine in the inverse electron demand Diels-Alder (IEDDA) reaction. Tetrazine ligation is a method of biological combination that can react quickly without catalysis. Tetrazine ligation is a rapid reaction with a second-order rate constant of 2000 M-1•S-1 (9:1 methanol/water), which allows modification of biomolecules at very low concentrations. This reaction can proceed at very high speeds in aqueous media. The reaction was also carried out in aqueous medium using trans-cyclooctene (TCO) or norbornene as a dienophile at a secondary rate of 1 M-1•S-1. Tetrazine ligation has been applied to label living cells and other biological entities,chemistry, biomedicine and other fields.
Applications of Tetrazine Ligation
Tetrazine ligation can be used to rapidly construct radionuclide labeled probes, which have been applied to label living cells and other biological entities. For example, Devaraj Neal K. reported that photoactivated tetrazine achieves accurate bioorthogonal chemistry of living cells.[1] Bioorthogonal cycloaddition reactions between tetrazines and strain dienophiles are widely used for labeling proteins, lipids, and glycans because of their extremely rapid kinetics. However, controlling such chemical reactions with a high degree of spatial and temporal precision in living mammalian cells remains a challenge. In this research work, the researchers demonstrated a versatile photoactivation method to form tetra-nitrogen by trapping dihydrotetra-nitrogen with light. Photocuring (dihydrotetra-nitrogen is trapped by light), followed by spontaneous conversion to active tetrazine, bioorthogonal cycloaddition reactions with dienophile substances such as TCO can be rapidly completed in living cells.
Application in Fluorescent Probe
At present, bioorthogonal fluorescent probe is gradually developed as an ideal tool for fluorescence imaging of living cells. Tetrazine bioorthogonal fluorescence enhanced probes play the dual role of bioorthogonal reaction unit and fluorescence quenching unit. The fluorescence "on" of these probes are mainly realized by IEDDA bioorthogonal reaction with inverse electron demand.
In 2010, Devaraj et al. reported for the first time the study of fluorescence switching on of tetrazine fluorescent probe based on IEDDA reaction.[2] Based on the Foster resonance energy transfer (FRET) principle, they connected 3-(4-benzyl amino)-1,2,4,5-tetrazine to the fluorophore BODIPY FL (Fig.1A) via unconjugated alkyl chains. The fluorescence quenched tetrazine probe was constructed, and its fluorescence was enhanced 15 times after reaction with dienophiles. Weissleder's group designed a series of highly efficient "on-off" tetrazine-BODIPY fluorescent probes (Fig.1B), which exhibited a 900-fold increase in fluorescence when reacted with dienophiles compared with the flexibly linked tetrazine-fluorophore conjugate.[3]
In contrast to the previous two mechanisms, the Par group reported an additional dark-state quenching mechanism.[4] They achieved relatively efficient dark state fluorescence quenching by strong electron coupling between tetrazine and Seoul-Fluor (SF) dye, and achieved a 67-fold fluorescence enhancement after the probe reacted with the dienophile TCO (emission wavelength: 493 nm, Fig.1C).
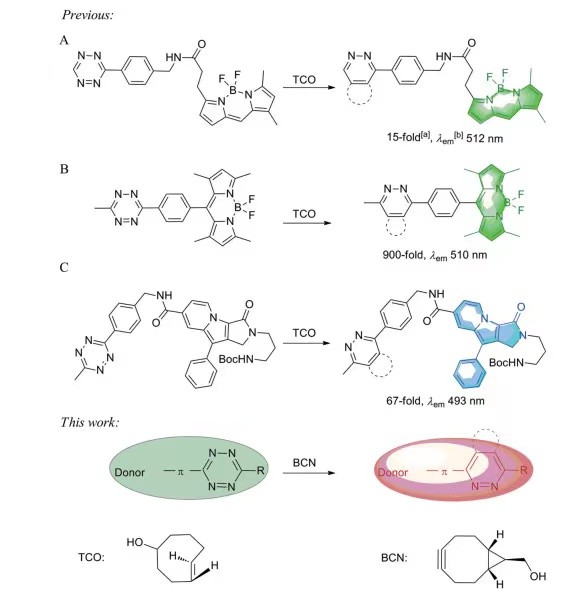 Fig.1 The process of forming fluorophore by bioorthogonal reactions of tetrazine [5]
Fig.1 The process of forming fluorophore by bioorthogonal reactions of tetrazine [5]
In addition, tetrazine ligation has many potential applications in the field of biomedicine. For example, the tetrazine quench fluorescent probe and prodrug vinyl ether CPT were coated in liposomes, and the liposomes were injected into mice through the tail vein to undergo IEDDA reaction (tetrazine ligation) for prodrug targeted release and tumor fluorescence imaging. Site-specific fluorescent labeling and imaging of intracellular proteins of interest (POI) can be performed by IEDDA cycloaddition reaction with tetrazine functionalized fluorophores after labeling cells with alkynes or alkene modified non-natural amino acids metabolism. Azide and TCO are scheduled to be located in specific subcellular locations and then undergo bioorthogonal reactions with bicyclo[6.1.0]non-4-yne (BCN) and tetrazine modified fluorescent dyes, respectively, which can simultaneously achieve multicolor labeling imaging of different targets. Tetrazine quenched fluorescent probe and photoinduced release were used to achieve the release of functional biomolecules under the dual control of visible light and bioorthogonal reaction.
Frequently Asked Questions (FAQ)
Tetrazine ligation is a bioorthogonal reaction in which a tetrazine reacts with a dienophile in an inverse electron demand Diels-Alder (IEDDA) reaction. This reaction is rapid and catalysis-free, allowing for efficient modification of biomolecules, especially in living systems, without interfering with natural biochemical processes.
The tetrazine reacts with a strained dienophile (such as trans-cyclooctene or norbornene) to form a stable triazine product. The reaction is highly efficient and can be used to modify biomolecules, such as proteins, lipids, and other biological entities.
Tetrazine ligation is widely used in biomolecule labeling, including proteins, lipids, and glycans. It’s also applied in fluorescence imaging, materials science, and the development of bioorthogonal probes for various biological assays.


References
- Luping Liu, Light-activated tetrazines enable precision live-cell bioorthogonal chemistry. Nat. Chem. 2022.
- Devaraj NK, Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew Chem Int Ed, 2010, 49: 2869-2872.
- Carlson JCT, BODIPY-tetrazine derivatives as superbright bioorthogonal turn-on probes. Angew Chem Int Ed, 2013, 52: 6917-6920.
- Meimetis LG, Ultrafluorogenic coumarin-tetrazine probes for real-time biological imaging. Angew Chem Int Ed, 2014, 53: 7531-7534.
- SU Dun-yan, Design and synthesis of tetrazine bioorthogonal fluorogenic probes. Acta Pharmaceutica Sinica, 2021, 56(4): 1086-1095.