Strain-Promoted Alkyne-Azide Cycloadditions (SPAAC)
As one of the most common click reactions, strain-promoted alkyne-azide cycloaddition (SPAAC) does not require the use of metal catalysts, reducing agents or stable ligands. On the contrary, the reaction uses the enthalpy released from the ring stress to cyclooctyne to form stable triazole.
What is SPAAC?
SPAAC can be carried out efficiently. On the one hand, the chemical potential energy of azide and alkyne substrates as reactants is very high. When cycloaddition reaction generates stable triazole, it can release more than 188 kJ/mol of heat, which satisfies the condition that reactants need high energy. On the other hand, azides and alkynes are difficult to react with biomolecules under reaction conditions, and they are inert to most other reaction reagents and solvents. In addition, alkynyl and azide groups have small molecular weights and weak polarities, which have little influence on the chemical properties of the connected structures, and meet the selectivity required in many applications such as biology and materials. Therefore, SPAAC takes advantage of the high ring tension of the cyclic alkyne itself, and the click reaction with chemical regioselectivity can occur without the participation of copper catalyst. It is a new and efficient azide alkyne cycloaddition reaction. (Figure 1)[1]
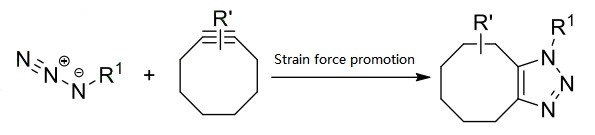 Fig.1 Reaction mechanism of SPAAC
Fig.1 Reaction mechanism of SPAAC
SPAAC can be applied to biological systems as a highly efficient bioorthogonal reaction. This reaction has good activity and selectivity under biological conditions and keeps good inertness to acidification, oxidation conditions and active amino acid molecules. However, this kind of reaction requires strict structure of monomer and is difficult to prepare.
Application of SPAAC
Because of the advantages of mild conditions, simple and high efficiency, and biological orthogonality, SPAAC has been widely used in many fields such as biomedicine and chemical materials, showing strong application prospects.
Pharmaceutical field
SPAAC has good regioselectivity. In addition to the fact that no impurities will be introduced during the directional binding process, the introduction of low molecular weight azides and cycloalkynes into macromolecules will not affect the expression of the effective energy of macromolecules after binding. In addition, SPAAC has the advantage that it can occur spontaneously under physiological conditions. Therefore, SPAAC can play a role in guiding specific binding in the field of medicine and has become an important tool in the field of medicine. In addition to the most important synthetic targeted anticancer drugs and playing a tracer role in pathological research, SPAAC can also be used for the synthesis and administration of conventional drugs, and even help to prepare highly active radiopharmaceuticals.
Application in life science
SPAAC has good bioorthogonality in the field of biomolecules. On the premise that copper catalyst is not required, the introduction of azides and cycloalkynes alone will not bring biological toxicity or affect the efficiency of biomolecules, so it can help achieve many synthesis or specific binding that were difficult to achieve before. Its good biological orthogonal reactivity enables it to react in living cells or tissues without interfering with the reaction of biological biochemical processes. For example, a series of hydrogels with controllable viscoelasticity, stiffness and degradation properties can be accurately constructed through SPAAC.
Application in material science
The characteristics of high efficiency and selectivity of SPAAC also have good application prospects in the field of materials science. This excellent property can be used for the preparation of films, coatings, adhesives, functional polymers and dendrimers, or for the surface modification and modification of materials. For example, SPAAC between azide functionalized gold nanoclusters and cyclooctyne has opened up a new platform for surface modification of nanoclusters after functional assembly.
Frequently Asked Questions (FAQ)
Strain-promoted alkyne-azide cycloaddition (SPAAC) is a metal-free click reaction that occurs between an alkyne and an azide group. The reaction leverages the ring strain of a cycloalkyne, which releases energy upon cyclization to form a stable triazole. This reaction does not require any metal catalysts, making it ideal for bioorthogonal applications, especially in biological environments.
SPAAC's bioorthogonality makes it an ideal tool for biomolecule labeling. It can be used to label proteins, lipids, and nucleic acids without disrupting biological processes, as it does not interfere with existing biological reactions. This allows for precise molecular tracking and visualization in live cells and tissues, aiding in the study of complex biological systems.
SPAAC is beneficial in drug development, particularly in the synthesis of targeted therapies. It allows for the efficient and specific binding of small molecules or macromolecules, without introducing impurities. Its ability to occur spontaneously under physiological conditions makes it an excellent tool for creating targeted drug delivery systems or for labeling drugs for diagnostic applications.


Reference
- Yiming Liao; et al. Strain-Promoted Azide-Alkyne Cycloaddition [J]. Progress in Chemistry. 2022, 1-16.