Fluorescent Labeling Technology
What is Fluorescent Labeling?
Fluorescent labeling is a biophysical term published in 2018. Fluorescent labeling is a method of selectively covalently binding or physically adsorbing a fluorescent dye (fluorescent group or fluorescein) to a certain group of the molecule (protein, peptide, etc.) to be studied, and uses the fluorescent properties of the fluorescent dye to provide information about the object being studied. The compound on which fluorescent labeling relies is called a fluorescent substance, which refers to a compound with a chemical structure of a conjugated double bond system. When irradiated by ultraviolet light or blue-violet light, it can be excited into an excited state, and when it returns to the ground state from the excited state, it emits fluorescence. The advantages such as no radioactive pollution and easy operation make the application of fluorescent labeling in many research fields increasingly widespread.
Common fluorophores
Fluorophores are generally functional groups that are excited by visible light (ultraviolet) irradiation. Common fluorescent substances include fluorescent pigments and other fluorescent substances.
Fluorescent pigments include:
- Fluorescein isothiocyanate (FITC): presents bright yellow-green fluorescence.
- Tetraethyl Rhodamine (RB200): orange-red fluorescence.
- Tetramethyl Rhodamine Isothiocyanate (TRITC): orange-red fluorescence.
- Phycoerythrin (R-RE): bright orange fluorescence.
Other fluorescent substances include lanthanide chelates and substances that produce fluorescence after the action of enzymes
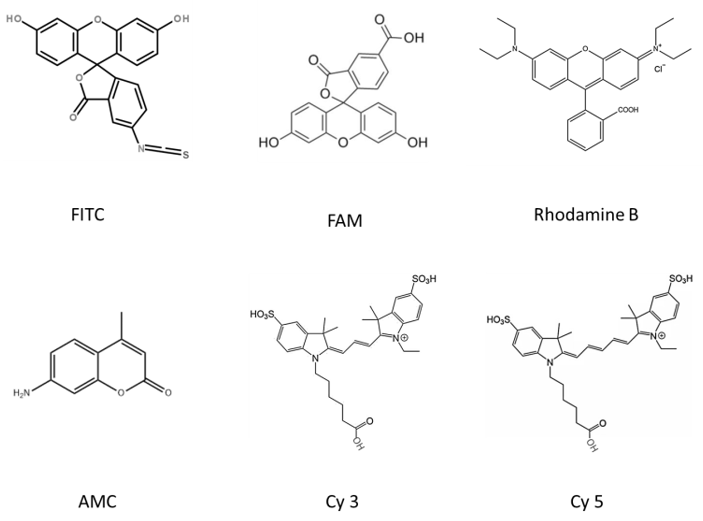 Figure 1. The chemical structure of common fluorescent substances (Drawn by Liudan).
Figure 1. The chemical structure of common fluorescent substances (Drawn by Liudan).
Examples of fluorescent labeling
FITC modification
Fluorescein isothiocyanate (FITC) has relatively high activity. Generally, it is easier to introduce this type of fluorophore during solid-phase synthesis than other fluoresceins, and there is no need to add activating reagents during the reaction. FITC modified peptides usually have two main forms:
(1) Connect FITC at the N-terminus of the entire peptide chain, and insert a molecule of Acp (6-aminocaproic acid) before FITC, also known as an alkyl spacer.
(2) One of the peptides in the entire peptide chain: The Lys side is linked into FITC, and the Lys side chain is a four-carbon straight-chain alkyl with the terminal -NH2, which directly plays a role in reducing steric hindrance.
AMC modification
7-Amino-4-methylcoumarin (AMC) is a widely used fluorescent labeling reagent, for example, trace determination of enzymes, identification of enzymes, preparation of laser dyes, etc. In addition, Coumarin-modified ubiquitin molecules are also important probes for studying the process of protein ubiquitination.
5-FAM modification
It is usually used in the application of laser confocal microscope and flow cytometry technology.
Rhodamine B (Rhodamine B) modification
Rhodamine B is one of many rhodamine dyes used for fluorescence determination.
5-TAMRA modification
5-TAMRA is one of the most popular orange fluorophores for labeling peptides and proteins.
Cy 3 and Cy 5 modification
Cy3 and Cy5 are dyes with high extinction coefficient. Therefore, they are particularly suitable for sensitive peptide localization experiments in cells. Their disadvantage is that they are very unstable in the environment of peptide synthesis, so the amount of synthesis is relatively low.
The role of fluorescent labeling technology
Fluorescent labeling technology also has a wide range of applications in the fields of protein function research and drug screening. People use fluorescently labeled peptides to detect the activity of target proteins, and the high-throughput activity screening methods developed by them are applied to drug screening and drug development of target proteins for disease treatment (for example, various kinases, phosphatases, peptidases, etc.). Fluorescence has a wide range of applications in the fields of biochemistry and medicine. People can attach fluorescent chemical groups to biological macromolecules through chemical reactions, and then sensitively detect these biological macromolecules by observing the fluorescence emitted by the tracer group. Common applications include: chain end termination method for automatic DNA sequencing; DNA detection; DNA microarray (biochip); flow cytometry (also known as fluorescence activated cell sorter, FACS); fluorescence microscopy imaging Technology, etc. Fluorescent labeling technology is also used to detect and analyze the molecular structure of DNA and proteins, especially the more complex biological macromolecules. For example, green fluorescent protein and related proteins have become important tools in biochemistry and cell biology research.
Frequently Asked Questions (FAQ)
Different fluorophores vary in their fluorescence intensity, stability, and wavelength range. For example, FITC offers a bright yellow-green fluorescence, while Cy3 and Cy5 are excellent for high-sensitivity applications like peptide localization in cells. Choosing the right fluorophore depends on the specific experimental needs, such as the detection method and the target sample.
Fluorescently labeled peptides or proteins can be used in high-throughput assays to screen for potential drug candidates by directly measuring biological activities such as enzyme activity or protein interactions. This method speeds up the process of identifying compounds that affect specific molecular targets, offering a significant advantage in drug discovery and development.
Fluorescent labeling is widely used to track and study protein-protein interactions in live cells or in vitro. By covalently attaching fluorescent dyes to specific proteins, researchers can observe and quantify interactions, providing crucial insights into cellular processes, such as signal transduction and gene regulation.
Fluorescent labeling enables the precise detection and analysis of nucleic acids (DNA/RNA) in various assays, such as DNA sequencing, microarray analysis, and PCR. The incorporation of fluorescent probes allows for highly sensitive and specific visualization of nucleic acid sequences, helping to identify mutations, gene expression, or other genetic features.


References
- Elena D. FLUORESCENCE LABELING[M]. 2019.
- Drummen, Gregor. Fluorescent Probes and Fluorescence (Microscopy) Techniques -Illuminating Biological and Biomedical Research[J]. Molecules, 2012, 17(12):14067-14090.
- Roth J, Bendayan M, Orci L. FITC-protein A-gold complex for light and electron microscopic immunocytochemistry[J]. Journal of Histochemistry & Cytochemistry Official Journal of the Histochemistry Society, 1980, 28(1):55.
- Sekar N. Rhodamine fluorophores €" functional applications[J]. Colourage, 2001.
- Alayed K M, Medeiros L J, Phan D, et al. Immunostaining for rapid diagnosis of acute promyelocytic leukemia with the tetramethyl rhodamine-5-isothiocyanate conjugated anti-promyelocytic leukemia monoclonal antibody PG-M3. [J]. Archives of Pathology & Laboratory Medicine, 2013, 137(11):1669-1673.
- Goodfellow M, Thomas E G, James A L. Characterisation of rhodococci using peptide hydrolase substrates based on 7-amino-4-methylcoumarin[J]. FEMS Microbiology Letters, 2010, 44(3):349-355.
- Roden M M, Lee K H, Panelli M C, et al. A novel cytolysis assay using fluorescent labeling and quantitative fluorescent scanning technology[J]. Journal of Immunological Methods, 1999, 226(1-2):29-41.
- Schuelke, Markus. An economic method for the fluorescent labeling of PCR fragments. [J]. Nature Biotechnology, 2000, 18(2):233-234.